A case of gastric duplication cyst diagnosed by double contrast enhanced ultrasound [January 2024]
June 20, 2024
Breaking the Barrier: Ultrasound Diagnosis of Infantile Hypertrophic Pyloric Stenosis [March 2024]
July 9, 2024SUBMIT YOUR CASE
EFSUMB invites submission of interesting cases for the website section 'Case of the Month'. All CoM submissions are eligible for selection for free registration at the next Euroson congress. Two cases that receive the most 'likes' in a year will receive free registration for the next EUROSON congress and the third most liked liked case will receive a cash prize of 100 EUR.
The eyes cannot see what the mind does not. Ultrasound based diagnosis of aorta-mesenteric clamp syndrome in the emergency department
Authors: Rareș Crăciun [1],[2], Cristiana Grapă [1],[2]
[1.]Department of Gastroenterology, ”Prof. Dr. O. Fodor” Regional Institute of Gastroenterology and Hepatology, Cluj-Napoca, Romania
[2.] Department of Internal Medicine, ”Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
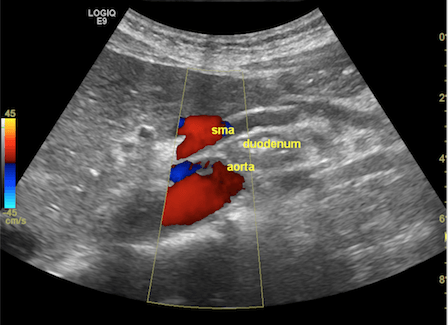
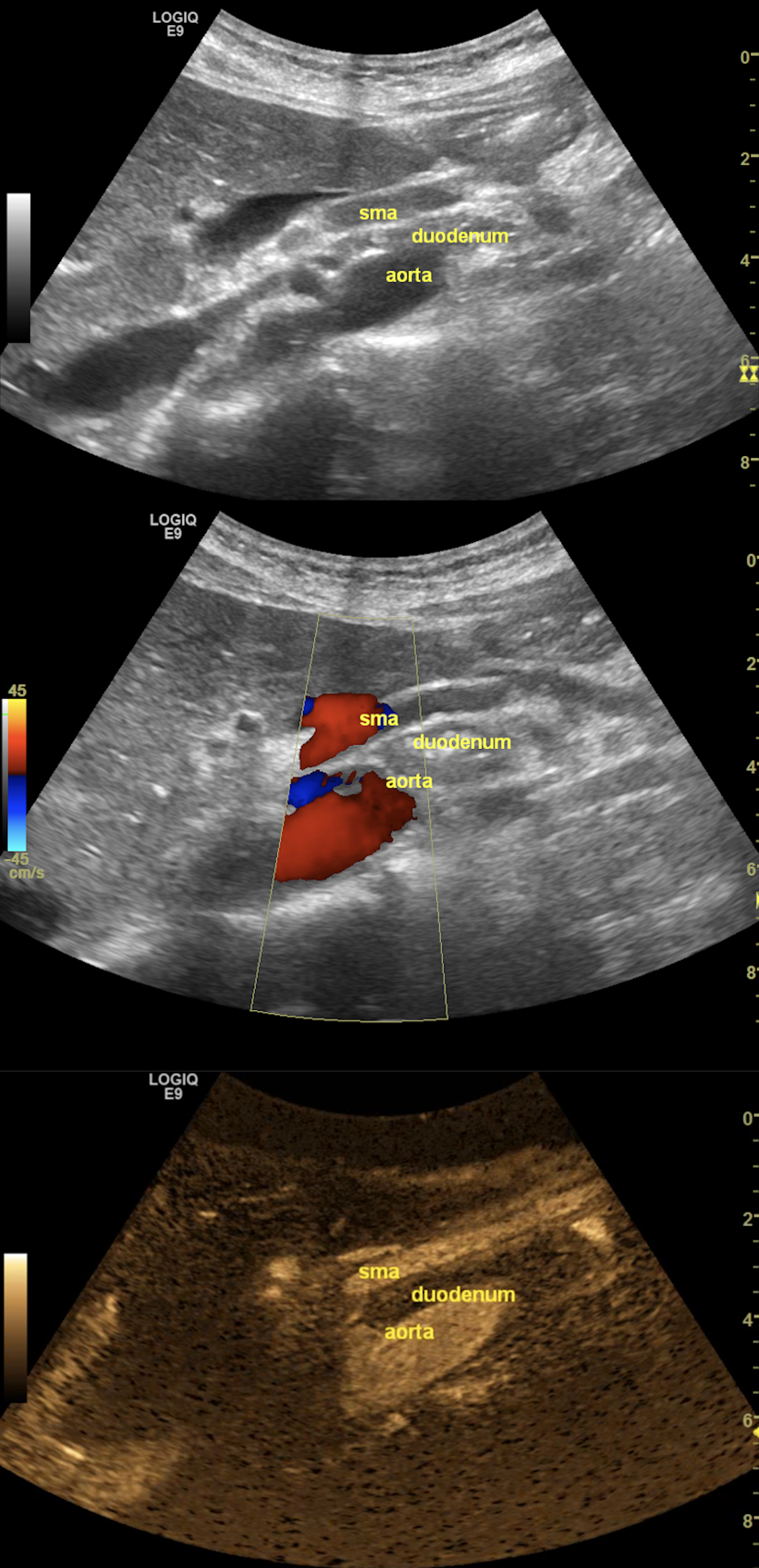
Figure 1. The grayscale ultrasonography reveals the aorto-mesenteric clamp, with the duodenum compressed by the superior mesenteric artery (SMA) and the abdominal aorta (up). The color Doppler mode confirms normal flow and patency (middle), while the B-Flow image singles out the arteries to emphasize the sharp aorto-mesenteric angle and the short distance between the two vessels (down).
In this report, we present the case of a 42-year-old female patient who presented to the ER with intense post-prandial epigastric pain without clinical response to any symptomatic treatment. The symptoms were accompanied by weight loss (15 kg in one year), bloating, and vomiting after large meals and had a significant impact on her quality of life. She was previously diagnosed with post-prandial distress syndrome following numerous consults in outpatient clinics, which included an extensive lab work-up, multiple abdominal ultrasonography (US) exams, and upper and lower gastrointestinal endoscopy, all unremarkable.
FAST abdominal US was performed per standard protocol, and no significant abnormalities were found at first glance. However, given her symptoms, which were precipitated by food intake, a closer look was given at the abdominal vasculature, focusing on potential signs of significant mesenteric atherosclerosis, given her history of smoking. When assessing the flow in the superior mesenteric artery (SMA), we observed a very tight angle of the SMA following its emergence from the abdominal aorta (AA), with an angle below 30°. In the opening of the angle between the two vessels, we observed the compressed duodenum and the left renal vein, which raised the suspicion of aorto-mesenteric clamp syndrome (Figure 1). The lack of significant aortic and mesenteric atherosclerosis, adequate mesenteric resistivity index, and the absence of indirect signs of intestinal ischemia further reinforced the most probable diagnosis.
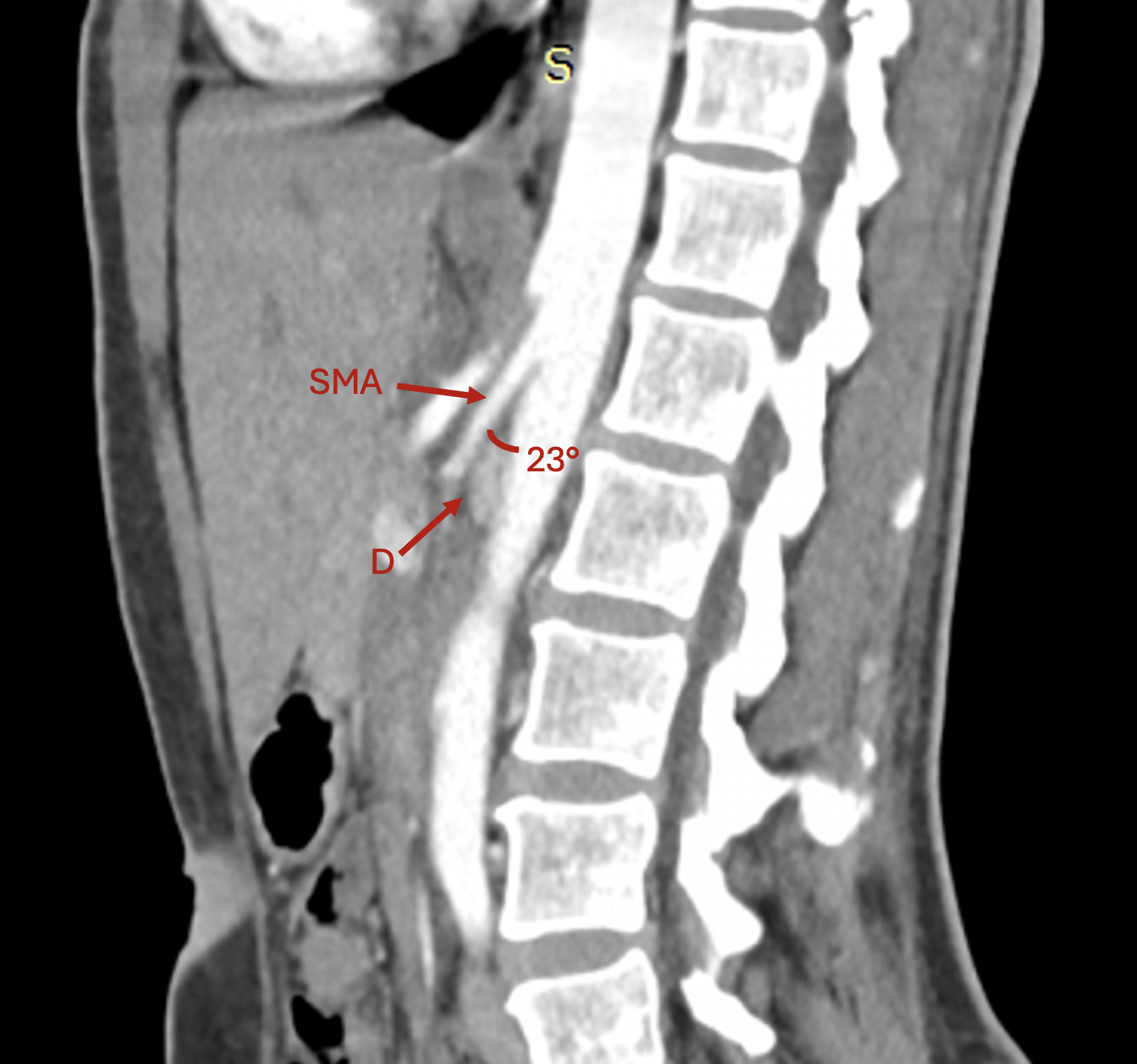
Contrast-enhanced computed tomography:
To confirm the diagnosis, a contrast-enhanced computed tomography (CE-CT) scan was performed, which revealed a 23° angle at the point of AMS emergence, which compressed the third part of the duodenum and led to mild gastric outlet obstruction, thus certifying the positive diagnosis of aorto-mesenteric clamp syndrome (Figure 2).
Aorto-mesenteric clamp syndrome, also known as SMA syndrome or Wilkie’s syndrome, is a rare condition defined by the compression of the third part of the duodenum and the left renal vein in a tight angle between the emergence of the SMA and the AA [1, 2]. While solid evidence regarding this condition is scarce and mostly based on small retrospective case series, the reported prevalence ranges between 0.01% and 0.33% [3,4]. From an anatomical standpoint, the angle between the SMA and the AA can vary widely in asymptomatic patients, from very sharp (10°) to obtuse (>140°), according to a recently published analysis on over three hundred asymptomatic patients undergoing CE-CT [5]. However, most available articles on this condition report a value < 30° on abdominal imaging [2,3,6]. Yet, given the lack of standardization, a clear cut-off value to define this condition has yet to be formally ascribed.
Clinical Perspective:
The aorto-mesenteric clamp syndrome is a differential that frequently flies under the radar in presentations with long-lasting severe abdominal pain. Thus, diagnosis is frequently delayed while the patient undergoes an otherwise extensive diagnostic work-up and fails multiple symptomatic therapy courses.
The clinical presentation can vary widely, from mild epigastric discomfort, early satiety, and post-prandial nausea or bloating, highly suggestive of functional disorders, to recurrent bouts of severe post-prandial pain mimicking intestinal ischemia or post-prandial vomiting suggestive of gastric outlet obstruction [6,7]. However, probably the most striking feature of this condition is the occurrence of weight loss despite preserved appetite and an otherwise unremarkable conventional work-up. In patients with an anatomical predisposition, namely a sharp SMA-AA angle, the loss of intraabdominal adipose tissue due to various factors diminishes the fat wrapping in the retroperitoneum, which provides a soft cushion for the duodenum and the left renal vein in the SMA-AA clamp. This trigger event generates a vicious cycle in which symptoms are usually exacerbated and further enhance weight loss [2].
In our case, a thorough US exam in the E.R. proved decisive for the diagnosis, which was further confirmed by a CE-CT scan. Of note, we would like to highlight that in most of the cases with aorto-mesenteric clamp syndrome, the patients are thin and the abdominal vasculature is easily visualized in normal conditions.
Therapy Planning:
Initial management often focuses on conservative measures, especially in patients with mild to moderate symptoms. These measures include nutritional support to address malnutrition and weight loss, which can exacerbate the condition. This often involves nasojejunal feeding to bypass the obstructed segment of the duodenum, high-calorie and high-protein diets, and, in some cases, parenteral nutrition. Positional therapy, such as encouraging the patient to lie in a left lateral decubitus, prone, or knee-chest position, can help alleviate symptoms by altering the anatomical relationship between the SMA and the aorta, thereby reducing compression on the duodenum [8–10].
When conservative management fails or is deemed insufficient, surgical intervention becomes necessary. Duodenojejunostomy is the most commonly performed surgical procedure, which involves creating a bypass around the obstructed segment of the duodenum. This procedure has a high success rate and is associated with significant symptomatic relief [11]. Another surgical option is the Strong’s procedure, which entails the mobilization of the duodenum (duodenojejunostomy), although it is less commonly performed due to variable outcomes. In certain cases, gastrojejunostomy or laparoscopic approaches may be considered, offering minimally invasive alternatives with reduced recovery times [2,7].
Our case was discussed on a multidisciplinary board, and aggressive nutritional therapy was selected as the first therapeutic choice. The patient was started on a soft, liquid, high-calorie (>45 kCal/kg of ideal body weight/day), high-protein diet (>1.5 g of protein/kg of ideal body weight/day).
Outcome:
The therapeutic decision resulted in a weight gain of approximately five kg in three months. From a clinical standpoint, the symptom burden was significantly reduced, albeit still present. Still, the quality of life was drastically improved, allowing the patient to perform her usual professional duties and daily activities.
Prognosis:
Overall, given the relatively rapid response to nutritional therapy, the expected prognosis is good, and a complete symptomatic remission is expected. In case of inadequate symptom control, according to the stepwise approach of this condition, a duodenojejunostomy will be proposed.
2. Proper imaging is crucial for diagnosis. Abdominal US, with a focus on vascular structures, can reveal a decreased angle between the superior mesenteric artery and the aorta, suggesting duodenal compression. CE-CT is the gold standard for confirming the diagnosis by providing detailed anatomical visualization of the compression and any resultant gastric outlet obstruction.
3. In most cases, nutritional therapy is sufficient to provide significant symptom relief. In case of inadequate control, patients can be referred to surgery, with the most common procedure being a duodenojejunostomy.
2. Merrett ND, Wilson RB, Cosman P, Biankin A V. Superior mesenteric artery syndrome: Diagnosis and treatment strategies. J Gastrointest Surg. 2009;13(2):287–92.
3. Agrawal GA, Johnson PT, Fishman EK. Multidetector row CT of superior mesenteric artery syndrome. J Clin Gastroenterol. 2007;41(1):62–5.
4. Ylinen P, Kinnunen J, Höckerstedt K. Superior mesenteric artery syndrome. A follow-up study of 16 operated patients. J Clin Gastroenterol. 1989 Aug;11(4):386-91. PMID: 2760427.
5. Hadi SS, Kareem TF, Kamal AM. Normal values of angle and distance between the superior mesenteric artery and aorta in Iraqi population: A single centre study. J Med Radiat Sci. 2022;69(2):191–7.
6. Kumar R, Jaiswal G, Bhargava A, Kundu J. Superior mesenteric artery syndrome: Diagnosis and management. Kathmandu Univ Med J. 2016;14(55):288–91.
7. Mandarry MT, Zhao L, Zhang C, Wei ZQ. A comprehensive review of superior mesenteric artery syndrome. Eur Surg - Acta Chir Austriaca. 2010;42(5):229–36.
8. Ciortescu I, Nemteanu R, Hincu C, Gheorghe L, Plesa A. An Underrated Diagnosis of Superior Mesenteric Artery Syndrome: A Case Report. Diagnostics. 2022;12:2159.
9. Karam A, Aziz T, Sarfaraz H, Karam AS, Karam AM, Khan NU. Superior mesenteric artery syndrome in a malnourished female: A rare cause of abdominal pain. Radiol Case Reports [Internet]. 2022;17(9):3165–7. Available from: https://doi.org/10.1016/j.radcr.2022.05.068
10. Forte A, Santarpia L, Venetucci P, Barbato A. Aorto-mesenteric compass syndrome (Wilkie’s syndrome) in the differential diagnosis of chronic abdominal pain. BMJ Case Rep. 2023;16(9):2024.
11. Kirby GC, Faulconer ER, Robinson SJ, Perry A, Downing R. Superior mesenteric artery syndrome: A single centre experience of laparoscopic duodenojejunostomy as the operation of choice. Ann R Coll Surg Engl. 2017;99(6):472–5.