- European Federation of Societies for Ultrasound in Medicine and Biology ~ Educating all for competence to practice ultrasound safely

(Ch:38) 50th Anniversary Booklet
March 6, 2017Paediatric CEUS
March 16, 2017Abdominal pseudocyst caused by peritoneal dialysis
Over a period of 6 years (2007 – 2013) a 60-year old man with chronic renal insufficiency be-cause of autosomal-dominant polycystic kidney disease (APDKD) was treated by continuous ambulatory peritoneal dialysis (CAPD). In the course of his treatment he experienced 2 culture proven peritonitic events (due to taenia saginata in 2009 and staphylococcus lentus in 2012) without evidence for tunnel infection. Clinical examination revealed no fever, a soft abdomen with no pain, no tenderness or resistance on palpation. Ultrasound of the abdomen showed the intraperitoneally located catheter (Fig 1).
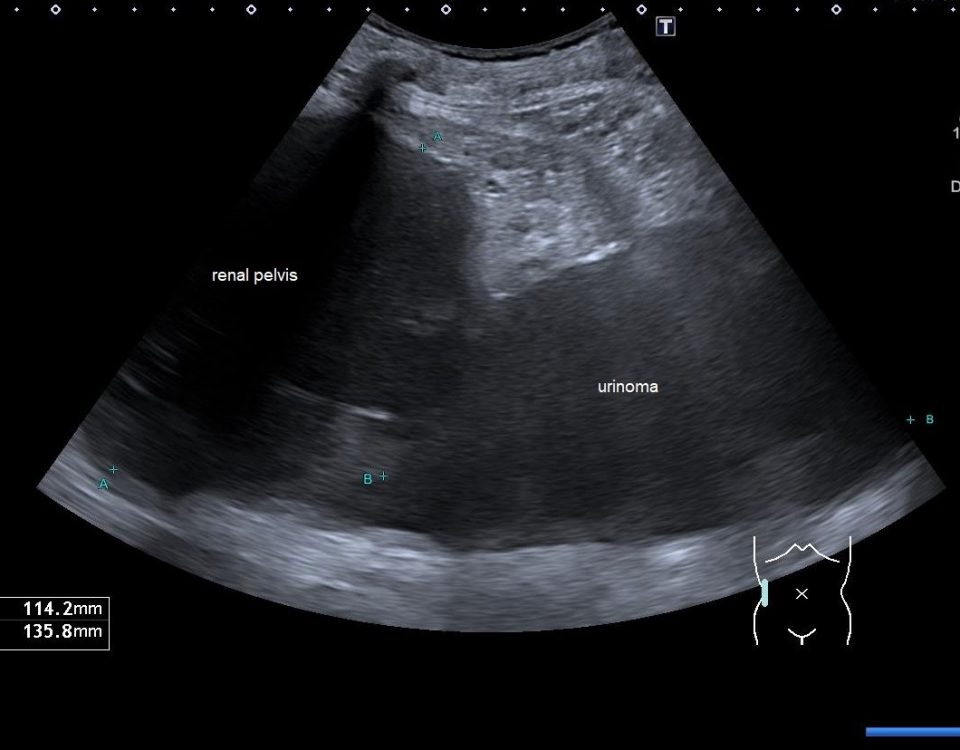
A large cystic lesion was detected on B-mode ultrasound with thick capsule wall (Fig 2).
After the intraperitoneally administration of SonoVue® the distribution of the contrast agent was evaluated. The entire pseudocystic lesion was rapidly and homogenously enhanced (Fig 3).
Contrast enhanced ultrasound (CEUS) with high frequency probe showed in the near field that the lesion was extraperitoneally located (Fig 4).
CT showed the instilled dialysate entrapped in a cyst that enclosed the inner tip of the Tenck-hoff catheter (Fig 5).
The suspected diagnosis was peritoneal pseudocyst. An exploratory laparotomy was per-formed followed by surgical removal of the catheter with excision of the pseudocystic wall. Histologic examination of the excised cyst showed postsurgical a much smaller diameter (5.5 x 3.5 cm in size) with smooth surface. Its wall was about 0.2 – 0.3 cm thick with grey and white colors. Histology revealed sclerotic tissue with reactive changes and fibrinous elements caused by the inflammatory pseudocapsule. No evidence of malignancy was found. To date, he is in good clinical condition with stable kidney function under hemodialysis therapy. The final diagnosis was peritoneal pseudocyst.
Intraperitoneal pseudocyst formation is an uncommon complication of peritonitis in patients on CAPD and also in patients with ventriculo-peritoneal shunts (the most successful method for managing hydrocephalus) [(4, 5)]. The main complications of peritoneal dialysis (PD) ther-apy include catheter dysfunction (mainly caused by leakage, obstruction or malposition), in-adequacy of dialysis because of ultrafiltration failure, infection complications caused by peri-tonitis or tunnel or exit-site infections and hernias [(4, 6)]. The exact cause and pathophysiolo-gy of abdominal pseudocysts is still debated [(5)]. Predisposing factors for pseudocyst for-mations are: bacterial infections and abdominal surgery. In the case of ventriculo-peritoneal associated abdominal pseudocysts multiple shunt revisions, silicon allergy and prior shunt in-fection are discussed [(6)]. The most common intra-abdominal response to infection is the sheathing of the peritoneal catheter. Abdominal pseudocysts are distinct from encapsulated peritoneal sclerosis (EPS) and represent a distinctive complication of PD. Formation of a pseu-docyst around the tip of an intraperitoneal catheter has been described previously as a rare (incidence, 1 % - 4 %) complication of ventriculo-peritoneal shunts in the treatment of hydro-cephalus [(7)]. In the case of simultaneous decrease in dialysis solution inflow and dialysate outflow combined with decreases in dialysis adequacy after peritonic events, one should keep in mind the possibility of a fibrous pseudocyst enclosing the intraperitoneal catheter. The clin-ical presentation may resemble that of an acute abdomen including abdominal pain with or without a palpable mass, abdominal distension and tenderness, nausea and vomiting, consti-pation, fever and signs of shunt malfunction [(8, 9)].
As a differential diagnosis, EPS is also a serious and rare complication of long-term PD thera-py with high mortality. EPS is associated with the duration of PD and the number of peritonitis events [(10)]. Pseudocyst formation has not been described so far in EPS. Mortality in EPS is high, often caused by malnutrition and sepsis, whereas the outcome in our patients was excel-lent. Taken together, these observations make EPS unlikely in our patient [(5)]. Some clinical features, such as abdominal pain, weight loss and elevated inflammatory markers levels may occur in EPS [(10)]. However EPS is defined by partial bowel obstruction and encapsulated bowel loops as a result of sclerotic thickening of peritoneal membrane, consisting of dense layers of fibro-connective tissue infiltrated by mononuclear cells. Bowel obstruction was not present in our patient and histological features of the abdominal pseudocyst were different from that of EPS.
Ultrasound (US) is an easy way for a quick and reliable diagnosis of abdominal pseudocysts. Pseudocysts can be differentiated from ascites by their characteristic displacement of the gas containing bowel loops using ultrasound and conventional abdominal X-ray as well as by com-puted tomography. The absence of shifting dullness is also a helpful sign for diagnosis [(8)]. US is able to demonstrate the presence of localized peritoneal fluid collections as well as its size, contents and relations of the catheter to the wall. Sonographic signs include an echofree mass with or without septations. Peritoneal pseudocysts can be visualized as smooth echo-free fluid collection with pseudo-membrane. Ultrasound can also be used to percutaneously drain the pseudocysts [(4)]. Sometimes ultrasound failed to delineate the cyst due to its large size, favouring the diagnosis of ascites secondary to sclerotic peritonitis.
CEUS applied intravenously is nowadays a well-established imaging modality in the evaluation of liver, kidney, gastrointestinal tract and vascular imaging [(11-16)]. CEUS could well differ-entiate cysts from solid or solid-cystic lesions. Besides the conventional intravenous injection of contrast agents SonoVue can be administered intraperitoneally showing the distribution of the contrast agent indicating the extent of the pseudocyst. CEUS examined with the high fre-quency probe could also clearly show if the lesion location was peritoneal or extraperitoneal. Herewith we discuss features of abdominal pseudocysts and the role of extravascular and in-tracavitary contrast enhanced ultrasound (evCEUS) in its diagnosis and differential diagnosis. Extravascular contrast enhanced ultrasound has been applied for many indications including percutaneous cholangiography and drainage [(17, 18)], abscess evaluation [(19, 20)], nephros-tomy [(21)] and examination of Zenker’s diverticulum [(22)] and perineal applications [(23, 24)]. We refer to other cases of the month from our group [www.efsumb.org]. Major ad-vantages of this method include the lack of radiation exposure, high detail resolution and real time imaging as the additional beneficial features of CEUS.
The treatment involves exploratory laparotomy followed by surgical removal of the catheter with or without excision of the pseudocyst wall [(25)]. The treatment in neurosurgery is either sonographically guided aspiration of the trapped fluid or surgical replacement of the ventricu-lo-peritoneal shunt [(4, 26)]. Infected cysts generally resolve with antibiotics and surgical management. In the case of ventriculo-peritoneal shunt associated pseudocysts obstruction of the distal catheter must be treated as an emergency because it can lead to a significant in-crease in intracranial pressure, resulting in associated complications that can cause consider-able morbidity and possibly death. Overall mortality and prognosis appear to be good, alt-hough in our patient, the pseudocyst led to conversion to hemodialysis therapy.
Medizinische Klinik 2
Caritas-Krankenhaus
Uhlandstr. 7
97980 Bad Mergentheim
Tel:+49 7931 58 2201
Email: christoph.dietrich@ckbm.de
1 Clinic of Gastroenterology, 2 Department of Pathology, 3Department of Radiology, University Hospital “Tsaritsa Yoanna – ISUL”, Medical University Sofia, Bulgaria.
2. Dietrich CF, Ignee A, Hocke M, Schreiber-Dietrich D, Greis C. Pitfalls and artefacts using contrast enhanced ultrasound. Z Gastroenterol 2011;49:350-356.
3. Cui XW, Ignee A, Hocke M, Seitz K, Schrade G, Dietrich CF. Prolonged heterogeneous liver enhancement on contrast-enhanced ultrasound. Ultraschall Med 2014;35:246-252.
4. Baer G, Wagner A, Selbach J, Otto M, Weiner SM. Abdominal pseudocysts following peritoneal dialysis-associated peritonitis: a report of 3 cases. Am J Kidney Dis 2010;55:e15-19.
5. Working Group on Neurosurgical Outcomes M, Woo PY, Wong HT, Pu JK, Wong WK, Wong LY, Lee MW, et al. Primary ventriculoperitoneal shunting outcomes: a multicentre clinical audit for shunt infection and its risk factors. Hong Kong Med J 2016;22:410-419.
6. Kawaguchi Y, Saito A, Kawanishi H, Nakayama M, Miyazaki M, Nakamoto H, Tranaeus A. Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit Dial Int 2005;25 Suppl 4:S83-95.
7. Rainov N, Schobess A, Heidecke V, Burkert W. Abdominal CSF pseudocysts in patients with ventriculo-peritoneal shunts. Report of fourteen cases and review of the literature. Acta Neurochir (Wien) 1994;127:73-78.
8. Sahpazova E, Ruso B, Kuzmanovska D. Intraperitoneal pseudocyst formation: complication of fungal peritonitis in continuous ambulatory peritoneal dialysis. Hippokratia 2007;11:219-220.
9. Namasivayam J. Intraperitoneal pseudocyst formation as a complication of continuous ambulatory peritoneal dialysis. Br J Radiol 1991;64:463-464.
10. Mihalache O, Buga C, Doran H, Catrina E, Bobirca F, Patrascu T. Encapsulating Peritoneal Sclerosis - A rare and serious complication of peritoneal dialysis: Case series. J Med Life 2014;7 Spec No. 3:8-12.
11. Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, Piscaglia F, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013;39:187-210.
12. Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, Piscaglia F, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 2013;34:11-29.
13. Piscaglia F, Nolsoe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012;33:33-59.
14. Braden B, Ignee A, Hocke M, Palmer RM, Dietrich C. Diagnostic value and clinical utility of contrast enhanced ultrasound in intestinal diseases. Dig Liver Dis 2010;42:667-674.
15. Dietrich CF, Lembcke B, Jenssen C, Hocke M, Ignee A, Hollerweger A. Intestinal Ultrasound in Rare Gastrointestinal Diseases, Update, Part 2. Ultraschall Med 2015;36:428-456.
16. Dietrich CF, Lembcke B, Jenssen C, Hocke M, Ignee A, Hollerweger A. Intestinal ultrasound in rare gastrointestinal diseases, update, part 1. Ultraschall Med 2014;35:400-421.
17. Ignee A, Baum U, Schuessler G, Dietrich CF. Contrast-enhanced ultrasound-guided percutaneous cholangiography and cholangiodrainage (CEUS-PTCD). Endoscopy 2009;41:725-726.
18. Ignee A, Cui X, Schuessler G, Dietrich CF. Percutaneous transhepatic cholangiography and drainage using extravascular contrast enhanced ultrasound. Z Gastroenterol 2015;53:385-390.
19. Ignee A, Jenssen C, Cui XW, Schuessler G, Dietrich CF. Intracavitary contrast-enhanced ultrasound in abscess drainage--feasibility and clinical value. Scand J Gastroenterol 2016;51:41-47.
20. Barreiros AP, Braden B, Schieferstein-Knauer C, Ignee A, Dietrich CF. Characteristics of intestinal tuberculosis in ultrasonographic techniques. Scand J Gastroenterol 2008;43:1224-1231.
21. Cui XW, Ignee A, Maros T, Straub B, Wen JG, Dietrich CF. Feasibility and Usefulness of Intra-Cavitary Contrast-Enhanced Ultrasound in Percutaneous Nephrostomy. Ultrasound Med Biol 2016;42:2180-2188.
22. Cui XW, Ignee A, Baum U, Dietrich CF. Feasibility and usefulness of using swallow contrast-enhanced ultrasound to diagnose Zenker's diverticulum: preliminary results. Ultrasound Med Biol 2015;41:975-981.
23. Barreiros AP, Hirche TO, Ignee A, Nurnberg D, Dietrich CF. Indications and limitations of perineal ultrasound examination. Scand J Gastroenterol 2010;45:764-765.
24. Dietrich CF, Barreiros AP, Nuernberg D, Schreiber-Dietrich DG, Ignee A. [Perianal ultrasound]. Z Gastroenterol 2008;46:625-630.
25. Popa F, Grigorean VT, Onose G, Popescu M, Strambu V, Sandu AM. Laparoscopic treatment of abdominal complications following ventriculoperitoneal shunt. J Med Life 2009;2:426-436.
26. Sharifa AD. Ventriculoperitoneal shunt with communicating peritoneal & subcutaneous pseudocysts formation. Int J Health Sci (Qassim) 2014;8:107-111.
Figure 2: A large cystic lesion was detected on B-mode ultrasound (a) with thick capsule wall (b). Displacement of the bowel gas pattern and absence of shifting dull-ness could be noticed. Conventional contrast enhanced ultrasound revealed the characteristic enhancement pattern (c).
Figure 3: After administered SonoVue intraperitoneally, we evaluated the extension of contrast agents. The whole cystic lesion was rapidly and heterogenous (a) and later homogenously enhancing (b).
Figure 4: Contrast enhanced ultrasound with high frequency probe showed the lesion was extraperitoneal (a) [see also Figure 1 and 2b]. Artefacts may be encoun-tered (b) [(1-3)].
Figure 5: CT showed the instilled dialysate entrapped in a cyst that enclosed the inner tip of the Tenckhoff catheter (a-c).


![Abdominal pseudocyst caused by peritoneal dialysis</br> [Mar 2017]](https://efsumb.org/wp-content/uploads/2020/11/march2017-figure2a-c.jpg)
![Abdominal pseudocyst caused by peritoneal dialysis</br> [Mar 2017]](https://efsumb.org/wp-content/uploads/2020/11/march2017-figure3a-b.jpg)
![Abdominal pseudocyst caused by peritoneal dialysis</br> [Mar 2017]](https://efsumb.org/wp-content/uploads/2020/11/march2017-figure4a-b.jpg)
![Abdominal pseudocyst caused by peritoneal dialysis</br> [Mar 2017]](https://efsumb.org/wp-content/uploads/2020/11/march2017-figure5a-c.jpg)