CEUS in non-abdominal organs
November 6, 2020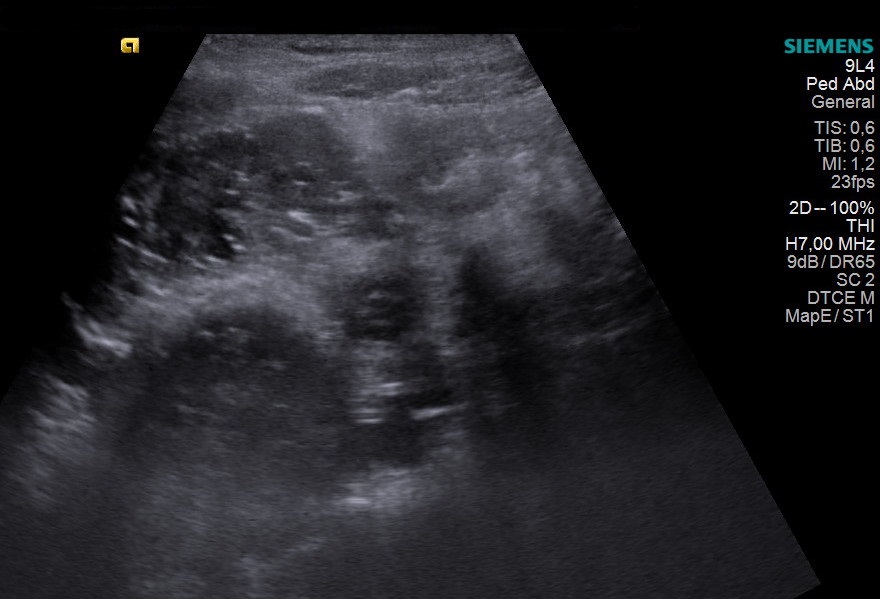
Student Image Challenge 73
November 12, 2020Congenital porto-systemic shunt as a cause of elevated manganese in adults
AUTHORS:
Olivia Goldberg & Kavita Shapriya (joint first authors), Andrew Logan, Adrian Lim
Olivia Goldberg & Kavita Shapriya (joint first authors), Andrew Logan, Adrian Lim
Figure 2B – Ultrasound video (see attachment) demonstrating right branch of the portal vein draining into the IVC
1Clinical history
A 40-year-old woman presented to the neurology outpatient department with a 5-year history of intermittent, vague sensory disturbances. At the time of presentation, her symptoms included generalised numbness and diaesthesia, tiredness, progressive exertional weakness and lumbar stiffness. She had developed intermittent blurred vision and bilateral ptosis, which required corrective surgery. Nerve conduction studies of the upper limbs and ocular muscles did not reveal any features of myasthenia gravis, Lambert-Eaton myasthenic syndrome or myopathy.
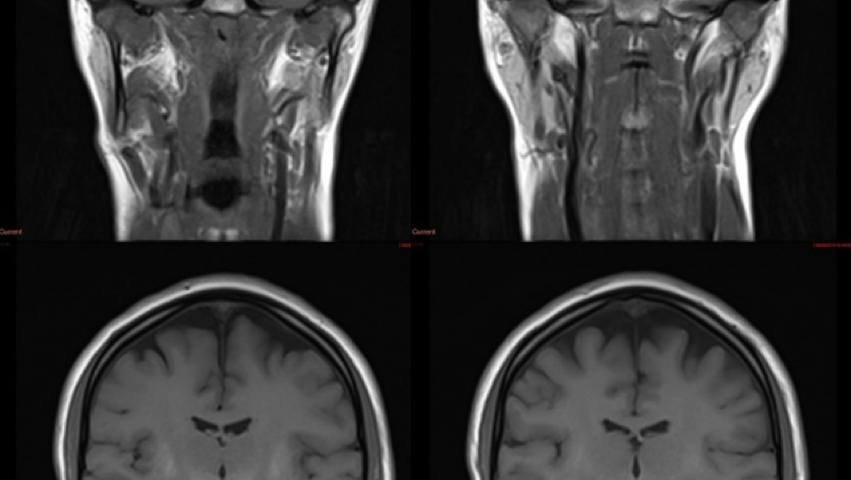
MRI of the spine demonstrated multilevel disco-vertebral degenerative changes with nerve root compression. MRI of the brain demonstrated bilateral, symmetrical T1 hyperintensity of the globus pallidus, substantia nigra, subthalamic nuclei, putamen and dentate nuclei. More subtle changes were seen in the white matter incantatory thalamic pathway and anterior pituitary gland. The appearances were highly suggestive of increased manganese deposition. Her blood manganese levels were elevated at 347nmol/L.
Based upon the MRI findings, she was investigated for any underlying causes of elevated manganese. On abdominal ultrasound, the liver had a normal size, outline, and echotexture. Interestingly, the right branch of the portal vein appeared to drain directly into the inferior vena cava. A congenital porto-systemic shunt was confirmed on a triple phase CT study of the liver which proved that the right branch of the portal vein drained directly into the IVC. There were no features to suggest chronic liver disease or collateralisation of the vessels.
MRI of the spine demonstrated multilevel disco-vertebral degenerative changes with nerve root compression. MRI of the brain demonstrated bilateral, symmetrical T1 hyperintensity of the globus pallidus, substantia nigra, subthalamic nuclei, putamen and dentate nuclei. More subtle changes were seen in the white matter incantatory thalamic pathway and anterior pituitary gland. The appearances were highly suggestive of increased manganese deposition. Her blood manganese levels were elevated at 347nmol/L.
Based upon the MRI findings, she was investigated for any underlying causes of elevated manganese. On abdominal ultrasound, the liver had a normal size, outline, and echotexture. Interestingly, the right branch of the portal vein appeared to drain directly into the inferior vena cava. A congenital porto-systemic shunt was confirmed on a triple phase CT study of the liver which proved that the right branch of the portal vein drained directly into the IVC. There were no features to suggest chronic liver disease or collateralisation of the vessels.
2Diagnosis
Elevated manganese due to a congenital porto-systemic shunt
3Discussion: Background
Portosystemic shunts can be congenital or acquired and can be divided into intrahepatic or extrahepatic shunts. Intrahepatic shunts are due to an abnormal intrahepatic connection between the portal vein and hepatic vein/inferior vena cava or a persistent patent ductus venosus, and often close spontaneously after birth. Extrahepatic shunts can be subdivided based on their anatomy(1) or the clinical presentation and liver histopathology, which guide subsequent treatment(2) . Extrahepatic shunts rarely close spontaneously. The risk of primary hepatocellular carcinoma in patients with extrahepatic shunts seems to be similar to that of liver cirrhosis(3) .
Manganese is a trace element that acts as a cofactor for diverse enzymes. The main source of manganese is dietary, with a daily intake of 0.9-10 mg(4) . Portosystemic shunting bypasses the normal first pass elimination of manganese, which is the principal route of manganese metabolism, and results in manganese excess(5) . Inhalational occupational exposure and total parenteral nutrition are also recognised causes of accumulation.
Manganese deposition has a predilection for the basal ganglia, specifically, the globus pallidus, striatum and substantia nigra(6) . The three routes of manganese entry into the brain are through the blood-brain barrier, cerebrospinal fluid and the olfactory tract(7) . The proposed mechanism of manganism is via mitochondrial oxidative stress, particularly in astrocytes(8) .
Manganese is a trace element that acts as a cofactor for diverse enzymes. The main source of manganese is dietary, with a daily intake of 0.9-10 mg(4) . Portosystemic shunting bypasses the normal first pass elimination of manganese, which is the principal route of manganese metabolism, and results in manganese excess(5) . Inhalational occupational exposure and total parenteral nutrition are also recognised causes of accumulation.
Manganese deposition has a predilection for the basal ganglia, specifically, the globus pallidus, striatum and substantia nigra(6) . The three routes of manganese entry into the brain are through the blood-brain barrier, cerebrospinal fluid and the olfactory tract(7) . The proposed mechanism of manganism is via mitochondrial oxidative stress, particularly in astrocytes(8) .
4Clinical perspective
Congenital portosystemic shunts (CPSS) are rare abnormalities that are thought to be due to a failure of the vitelline veins to obliterate. CPSS is usually diagnosed at birth or in children. Other anomalies associated with CPSS, which were absent in this case, encompass atrial and ventricular septal defects, tetralogy of Fallot and polysplenia, amongst others. This woman, however, presented as an adult, which suggests comparatively low shunt function.
Patients with congenital portosystemic shunts are often asymptomatic, and incidental findings on imaging are not uncommon. Symptomatic patients present with a wide spectrum of symptoms and complications which include hepatic encephalopathy, hepatopulmonary syndrome, and pulmonary hypertension caused by long-term portosystemic shunting. These complications are most often observed in children(9)(10) . Children may present with unexplained neurocognitive dysfunction, learning disabilities, extreme fatigability, seizures, and failure to thrive which is thought to be related to elevated ammonia levels(11) .
In CPSS-related manganese excess, neurotoxicity is the primary concern and encompasses cognitive, psychiatric, and Parkinsonian-like motor disturbances(12) . The earliest symptoms of neurotoxicity tend to be psychiatric in nature, such as emotional volatility or compulsive behaviour(13) . The motor effects documented include hypokinesia, rigidity and dystonia. Compared to Parkinsonism, a resting tremor is less common and levodopa does not induce a sustained improvement. Interestingly the patient presented with a mixture of sensory and motor symptoms, which raises the possibility of whether there are factors apart from manganese toxicity at play. Also unknown is the relative role of manganese compared to ammonia and other neurotoxic agents in the development of hepatic encephalopathy.
Patients with congenital portosystemic shunts are often asymptomatic, and incidental findings on imaging are not uncommon. Symptomatic patients present with a wide spectrum of symptoms and complications which include hepatic encephalopathy, hepatopulmonary syndrome, and pulmonary hypertension caused by long-term portosystemic shunting. These complications are most often observed in children(9)(10) . Children may present with unexplained neurocognitive dysfunction, learning disabilities, extreme fatigability, seizures, and failure to thrive which is thought to be related to elevated ammonia levels(11) .
In CPSS-related manganese excess, neurotoxicity is the primary concern and encompasses cognitive, psychiatric, and Parkinsonian-like motor disturbances(12) . The earliest symptoms of neurotoxicity tend to be psychiatric in nature, such as emotional volatility or compulsive behaviour(13) . The motor effects documented include hypokinesia, rigidity and dystonia. Compared to Parkinsonism, a resting tremor is less common and levodopa does not induce a sustained improvement. Interestingly the patient presented with a mixture of sensory and motor symptoms, which raises the possibility of whether there are factors apart from manganese toxicity at play. Also unknown is the relative role of manganese compared to ammonia and other neurotoxic agents in the development of hepatic encephalopathy.
5Therapy Planning
To date, there is no protective strategy against manganese neurotoxicity. A recent study in mice models showed taurine supplementation alleviated manganese-induced locomotor deficit, reduced oxidative stress biomarkers and preserved brain tissue mitochondrial functionality(14) . Similar results have been shown with carnosine(15) , quercetin(16) and vinpocetine(17) in animal models. Current treatment strategies for manganese toxicity combine calcium sodium edetate chelation therapy to reduce the amount of manganese load and iron supplementation to reduce manganese binding to protein(18) .
The most effective method of managing elevated manganese is to treat the underlying cause. There is no standard approach to treatment of porto-systemic shunts and strategies depend on the type of shunt, location and degree of function, patient age, and the severity of symptoms and complications. For congenital intrahepatic shunts, conservative management is often adopted for the first year since spontaneous closure is expected. Current opinion favours intervening surgically if the shunt persists after the first year, before complications develop(19) . Congenital extrahepatic shunts rarely close spontaneously and surgical treatment is often adopted early. Interventional radiology plays an important role in the preoperative evaluation with an occlusion test to assess the anatomy, as well as to measure portal pressure after treatment(20) . Endovascular closure is the first option for treatment when possible and includes embolization with various materials such as coils, detachable balloons or vascular plugs. Surgical options include ligation for larger shunts or those that have recurred after embolization, or liver resection/ transplant for cases of large extrahepatic or multifocal shunts.
The most effective method of managing elevated manganese is to treat the underlying cause. There is no standard approach to treatment of porto-systemic shunts and strategies depend on the type of shunt, location and degree of function, patient age, and the severity of symptoms and complications. For congenital intrahepatic shunts, conservative management is often adopted for the first year since spontaneous closure is expected. Current opinion favours intervening surgically if the shunt persists after the first year, before complications develop(19) . Congenital extrahepatic shunts rarely close spontaneously and surgical treatment is often adopted early. Interventional radiology plays an important role in the preoperative evaluation with an occlusion test to assess the anatomy, as well as to measure portal pressure after treatment(20) . Endovascular closure is the first option for treatment when possible and includes embolization with various materials such as coils, detachable balloons or vascular plugs. Surgical options include ligation for larger shunts or those that have recurred after embolization, or liver resection/ transplant for cases of large extrahepatic or multifocal shunts.
6Outcome
The patient is awaiting an MRCP to evaluate the portosystemic shunt and to guide further management.
7Prognosis
The outcome of intrahepatic portosystemic shunts diagnosed prenatally is good in the majority of cases(21) . Many adults who are diagnosed with a portosystemic shunt are asymptomatic. For those who are symptomatic, definitive treatment (endovascular or surgical) has been shown to reverse manganese deposition in the brain(22) and can normalise elevated levels of ammonia and manganese, thereby preventing neurotoxicity and long-term sequelae(23) .
8Teaching Points
1. Congenital portosystemic shunts are rare; this is an unusual case in an adult without other developmental anomalies.
2. Porto-systemic shunts are an important cause of elevated manganese in adults
3. Ultrasound studies play an important role in the diagnosis of porto-systemic shunts.
4. Interventional endovascular procedures provide initial definitive treatment of congenital porto-systemic shunts.
5. Early identification of portosystemic shunts, and subsequent monitoring and intervention, can reverse manganese-related T1 hyperintensity on MRI brain, and prevent long-term sequelae and neurotoxicity.
2. Porto-systemic shunts are an important cause of elevated manganese in adults
3. Ultrasound studies play an important role in the diagnosis of porto-systemic shunts.
4. Interventional endovascular procedures provide initial definitive treatment of congenital porto-systemic shunts.
5. Early identification of portosystemic shunts, and subsequent monitoring and intervention, can reverse manganese-related T1 hyperintensity on MRI brain, and prevent long-term sequelae and neurotoxicity.
9References
1. Stringer MD. The clinical anatomy of congenital portosystemic venous shunts. Clin Anat. 2008;21(2):147-157. doi:10.1002/ca.20574
2. Papamichail M, Ali A, Quaglia A, Karani J, Heaton N. Liver resection for the treatment of a congenital intrahepatic portosystemic venous shunt. Hepatobiliary Pancreat Dis Int. 2016;15(3):329-333. doi:10.1016/s1499-3872(16)60067-x
3. Konstas AA, Digumarthy SR, Avery LL, et al. Congenital portosystemic shunts: imaging findings and clinical presentations in 11 patients. Eur J Radiol. 2011;80(2):175-181. doi:10.1016/j.ejrad.2009.12.031
4. Chen P, Bornhorst J, Aschner M. Manganese metabolism in humans. Front Biosci (Landmark Ed). 2018;23:1655-1679. Published 2018 Mar 1. doi:10.2741/4665
5. Layrargues GP, Rose C, Spahr L, Zayed J, Normandin L, Butterworth RF. Role of manganese in the pathogenesis of portal-systemic encephalopathy. Metab Brain Dis. 1998;13(4):311-317. doi:10.1023/a:1020636809063
6. Rose C, Butterworth RF, Zayed J, et al. Manganese deposition in basal ganglia structures results from both portal-systemic shunting and liver dysfunction. Gastroenterology. 1999;117(3):640-644. doi:10.1016/s0016-5085(99)70457-9
7. Uchino A, Noguchi T, Nomiyama K, et al. Manganese accumulation in the brain: MR imaging. Neuroradiology. 2007;49(9):715-720. doi:10.1007/s00234-007-0243-z
8. Harischandra DS, Ghaisas S, Zenitsky G, et al. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front Neurosci. 2019;13:654. Published 2019 Jun 26. doi:10.3389/fnins.2019.00654
9. Sokollik C, Bandsma RH, Gana JC, van den Heuvel M, Ling SC. Congenital portosystemic shunt: characterization of a multisystem disease. J Pediatr Gastroenterol Nutr. 2013;56(6):675-681. doi:10.1097/MPG.0b013e31828b3750
10. Grimaldi C, Monti L, Falappa P, d'Ambrosio G, Manca A, de Ville de Goyet J. Congenital intrahepatic portohepatic shunt managed by interventional radiologic occlusion: a case report and literature review. J Pediatr Surg. 2012;47(2):e27-e31. doi:10.1016/j.jpedsurg.2011.10.079
11. Stringer MD. The clinical anatomy of congenital portosystemic venous shunts. Clin Anat. 2008;21(2):147-157. doi:10.1002/ca.20574
12. Roels HA, Bowler RM, Kim Y, et al. Manganese exposure and cognitive deficits: a growing concern for manganese neurotoxicity. Neurotoxicology. 2012;33(4):872-880. doi:10.1016/j.neuro.2012.03.009
13. Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115-128. doi:10.1196/annals.1306.009
14. Ommati MM, Heidari R, Ghanbarinejad V, Abdoli N, Niknahad H. Taurine Treatment Provides Neuroprotection in a Mouse Model of Manganism. Biol Trace Elem Res. 2019;190(2):384-395. doi:10.1007/s12011-018-1552-2
15. Ommati MM, Heidari R, Ghanbarinejad V, Aminian A, Abdoli N, Niknahad H. The neuroprotective properties of carnosine in a mouse model of manganism is mediated via mitochondria regulating and antioxidative mechanisms [published online ahead of print, 2019 Mar 11]. Nutr Neurosci. 2019;1-13. doi:10.1080/1028415X.2018.1552399
16. Bahar E, Kim JY, Yoon H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, iNOS/NF-κB and HO-1/Nrf2 Pathways. Int J Mol Sci. 2017;18(9):1989. Published 2017 Sep 15. doi:10.3390/ijms18091989
17. Bora S, Erdogan MA, Armagan G, Sevgili E, Dagcı T. Erratum to: Vinpocetine and Vasoactive Intestinal Peptide Attenuate Manganese-Induced Toxicity in NE-4C Cells. Biol Trace Elem Res. 2017;175(1):236. doi:10.1007/s12011-016-0775-3
18. Tuschl K, Mills PB, Clayton PT. Manganese and the brain. Int Rev Neurobiol. 2013;110:277-312. doi:10.1016/B978-0-12-410502-7.00013-2
19. Papamichail M, Pizanias M, Heaton N. Congenital portosystemic venous shunt. Eur J Pediatr. 2018;177(3):285-294. doi:10.1007/s00431-017-3058-x
20. Franchi-Abella S, Gonzales E, Ackermann O, et al. Congenital portosystemic shunts: diagnosis and treatment. Abdom Radiol (NY). 2018;43(8):2023-2036. doi:10.1007/s00261-018-1619-8
21. Francois B, Gottrand F, Lachaux A, Boyer C, Benoit B, De Smet S. Outcome of intrahepatic portosystemic shunt diagnosed prenatally. Eur J Pediatr. 2017;176(12):1613-1618. doi:10.1007/s00431-017-3013-x
22. Taguchi Y, Takashima S, Hirade S, Inoue H. Rinsho Shinkeigaku. 1999;39(5):565-569.
23. Takemoto R, Yamamura K, Nagata H, et al. Disappearance of globus pallidum lesions in T1-weighted magnetic resonance images after ligation of congenital portosystemic venous shunt. Pediatr Neonatol. 2017;58(5):465-466. doi:10.1016/j.pedneo.2017.05.001
2. Papamichail M, Ali A, Quaglia A, Karani J, Heaton N. Liver resection for the treatment of a congenital intrahepatic portosystemic venous shunt. Hepatobiliary Pancreat Dis Int. 2016;15(3):329-333. doi:10.1016/s1499-3872(16)60067-x
3. Konstas AA, Digumarthy SR, Avery LL, et al. Congenital portosystemic shunts: imaging findings and clinical presentations in 11 patients. Eur J Radiol. 2011;80(2):175-181. doi:10.1016/j.ejrad.2009.12.031
4. Chen P, Bornhorst J, Aschner M. Manganese metabolism in humans. Front Biosci (Landmark Ed). 2018;23:1655-1679. Published 2018 Mar 1. doi:10.2741/4665
5. Layrargues GP, Rose C, Spahr L, Zayed J, Normandin L, Butterworth RF. Role of manganese in the pathogenesis of portal-systemic encephalopathy. Metab Brain Dis. 1998;13(4):311-317. doi:10.1023/a:1020636809063
6. Rose C, Butterworth RF, Zayed J, et al. Manganese deposition in basal ganglia structures results from both portal-systemic shunting and liver dysfunction. Gastroenterology. 1999;117(3):640-644. doi:10.1016/s0016-5085(99)70457-9
7. Uchino A, Noguchi T, Nomiyama K, et al. Manganese accumulation in the brain: MR imaging. Neuroradiology. 2007;49(9):715-720. doi:10.1007/s00234-007-0243-z
8. Harischandra DS, Ghaisas S, Zenitsky G, et al. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front Neurosci. 2019;13:654. Published 2019 Jun 26. doi:10.3389/fnins.2019.00654
9. Sokollik C, Bandsma RH, Gana JC, van den Heuvel M, Ling SC. Congenital portosystemic shunt: characterization of a multisystem disease. J Pediatr Gastroenterol Nutr. 2013;56(6):675-681. doi:10.1097/MPG.0b013e31828b3750
10. Grimaldi C, Monti L, Falappa P, d'Ambrosio G, Manca A, de Ville de Goyet J. Congenital intrahepatic portohepatic shunt managed by interventional radiologic occlusion: a case report and literature review. J Pediatr Surg. 2012;47(2):e27-e31. doi:10.1016/j.jpedsurg.2011.10.079
11. Stringer MD. The clinical anatomy of congenital portosystemic venous shunts. Clin Anat. 2008;21(2):147-157. doi:10.1002/ca.20574
12. Roels HA, Bowler RM, Kim Y, et al. Manganese exposure and cognitive deficits: a growing concern for manganese neurotoxicity. Neurotoxicology. 2012;33(4):872-880. doi:10.1016/j.neuro.2012.03.009
13. Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115-128. doi:10.1196/annals.1306.009
14. Ommati MM, Heidari R, Ghanbarinejad V, Abdoli N, Niknahad H. Taurine Treatment Provides Neuroprotection in a Mouse Model of Manganism. Biol Trace Elem Res. 2019;190(2):384-395. doi:10.1007/s12011-018-1552-2
15. Ommati MM, Heidari R, Ghanbarinejad V, Aminian A, Abdoli N, Niknahad H. The neuroprotective properties of carnosine in a mouse model of manganism is mediated via mitochondria regulating and antioxidative mechanisms [published online ahead of print, 2019 Mar 11]. Nutr Neurosci. 2019;1-13. doi:10.1080/1028415X.2018.1552399
16. Bahar E, Kim JY, Yoon H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, iNOS/NF-κB and HO-1/Nrf2 Pathways. Int J Mol Sci. 2017;18(9):1989. Published 2017 Sep 15. doi:10.3390/ijms18091989
17. Bora S, Erdogan MA, Armagan G, Sevgili E, Dagcı T. Erratum to: Vinpocetine and Vasoactive Intestinal Peptide Attenuate Manganese-Induced Toxicity in NE-4C Cells. Biol Trace Elem Res. 2017;175(1):236. doi:10.1007/s12011-016-0775-3
18. Tuschl K, Mills PB, Clayton PT. Manganese and the brain. Int Rev Neurobiol. 2013;110:277-312. doi:10.1016/B978-0-12-410502-7.00013-2
19. Papamichail M, Pizanias M, Heaton N. Congenital portosystemic venous shunt. Eur J Pediatr. 2018;177(3):285-294. doi:10.1007/s00431-017-3058-x
20. Franchi-Abella S, Gonzales E, Ackermann O, et al. Congenital portosystemic shunts: diagnosis and treatment. Abdom Radiol (NY). 2018;43(8):2023-2036. doi:10.1007/s00261-018-1619-8
21. Francois B, Gottrand F, Lachaux A, Boyer C, Benoit B, De Smet S. Outcome of intrahepatic portosystemic shunt diagnosed prenatally. Eur J Pediatr. 2017;176(12):1613-1618. doi:10.1007/s00431-017-3013-x
22. Taguchi Y, Takashima S, Hirade S, Inoue H. Rinsho Shinkeigaku. 1999;39(5):565-569.
23. Takemoto R, Yamamura K, Nagata H, et al. Disappearance of globus pallidum lesions in T1-weighted magnetic resonance images after ligation of congenital portosystemic venous shunt. Pediatr Neonatol. 2017;58(5):465-466. doi:10.1016/j.pedneo.2017.05.001


![Congenital porto-systemic shunt as a cause of elevated manganese in adults </br> [Oct/Nov 2020]](https://efsumb.org/wp-content/uploads/2020/11/oct2020-fig1.png)
![Congenital porto-systemic shunt as a cause of elevated manganese in adults </br> [Oct/Nov 2020]](https://efsumb.org/wp-content/uploads/2020/11/oct2020-fig02.png)
![Congenital porto-systemic shunt as a cause of elevated manganese in adults </br> [Oct/Nov 2020]](https://efsumb.org/wp-content/uploads/2020/11/oct2020-fig3.png)