Student Image Challenge 104
March 27, 2024
Distal pancreatic stent migration: Could POCUS assist in identifying perforation in primary care? [September 2023]
April 26, 2024SUBMIT YOUR CASE
EFSUMB invites submission of interesting cases for the website section 'Case of the Month'. All CoM submissions are eligible for selection for free registration at the next Euroson congress. Two cases that receive the most 'likes' in a year will receive free registration for the next EUROSON congress and the third most liked liked case will receive a cash prize of 100 EUR.
Unclear lesion of the abdominal wall
Authors: Kaiser Ulrich[1], Jung Ernst Michael[2]
1 Medical Clinic and Polyclinic III, University Hospital Regensburg, Regensburg, Germany
2 Institute for Diagnostic Radiology and Interdisciplinary Ultrasound, University Hospital Regensburg, Regensburg, Germany
1Clinical History
Sonography is playing an increasingly important role in the diagnosis of soft tissue masses. Such masses may be palpable or incidental findings hidden in depth by other imaging procedures. For example, in magnetic resonance imaging (MRI), soft tissue masses with contrast enhancement are usually considered suspicious. If no primary tumor entities can be identified in the affected examination area, sonography may be necessary for a final assessment using ultrasound-guided biopsy. In young patients, the suspected diagnosis is usually a sarcoma. The assessment of the MRI findings raised the suspicion of a potential sarcoma of the abdominal wall.
Within a short period of time, a young female patient noticed increasing, painful and palpable resistance in the lower abdomen, centered in the rectus abdominis muscle. Palpable lymph nodes were denied by the patient. There were also no indications of an acute inflammatory abdominal event. There was a history of a caesarean section. The patient denied further surgical interventions. There was no laboratory evidence of acute inflammation. The clinical examination did not indicate appendicitis or colitis.
Within a short period of time, a young female patient noticed increasing, painful and palpable resistance in the lower abdomen, centered in the rectus abdominis muscle. Palpable lymph nodes were denied by the patient. There were also no indications of an acute inflammatory abdominal event. There was a history of a caesarean section. The patient denied further surgical interventions. There was no laboratory evidence of acute inflammation. The clinical examination did not indicate appendicitis or colitis.
2Image findings
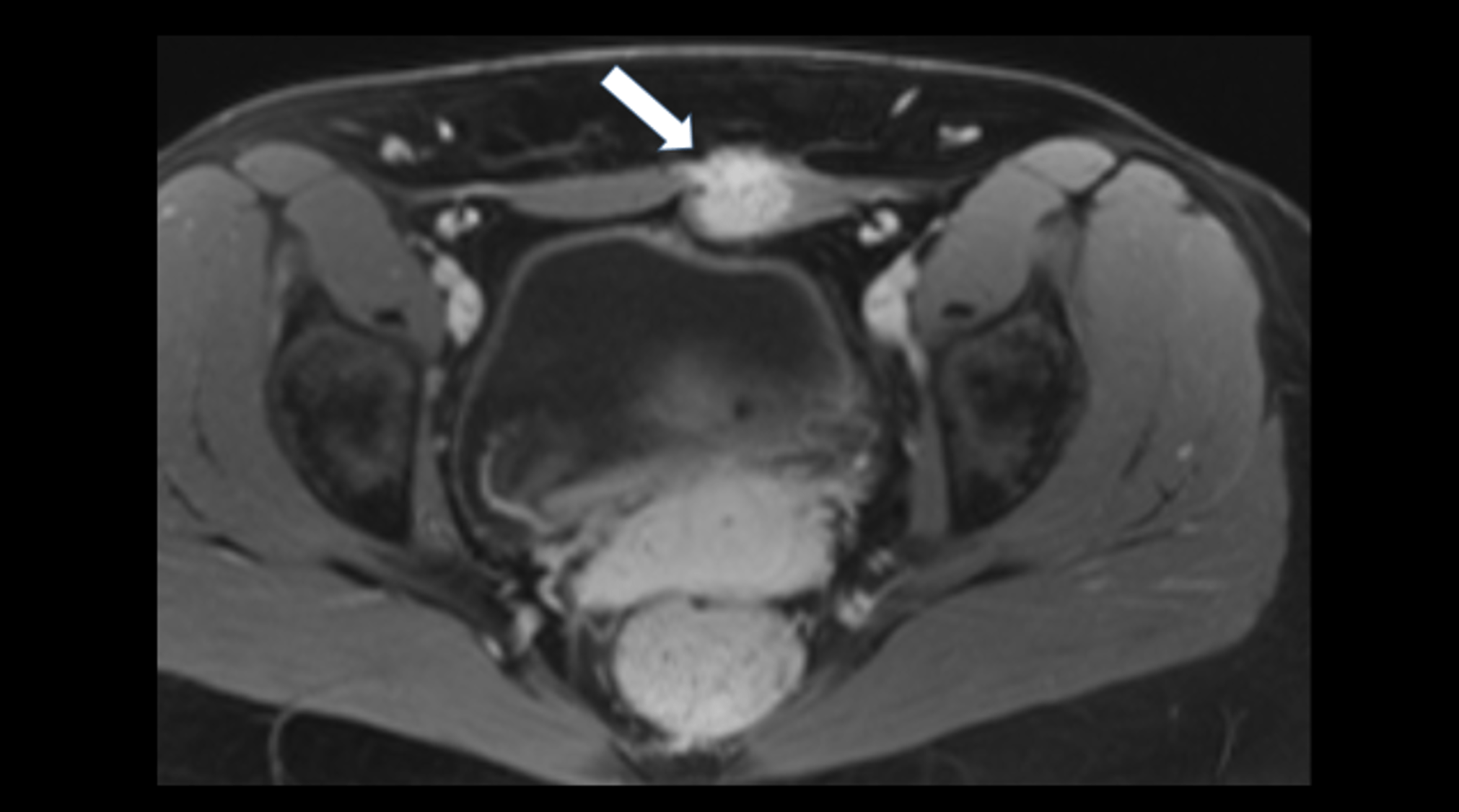
Abdominal ultrasonography (Figures 1-2):
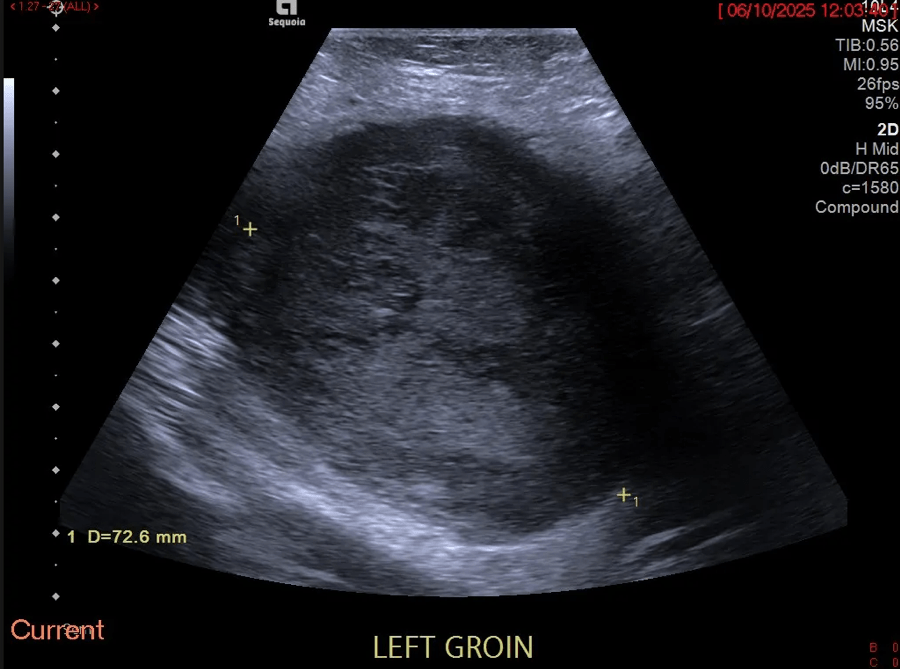
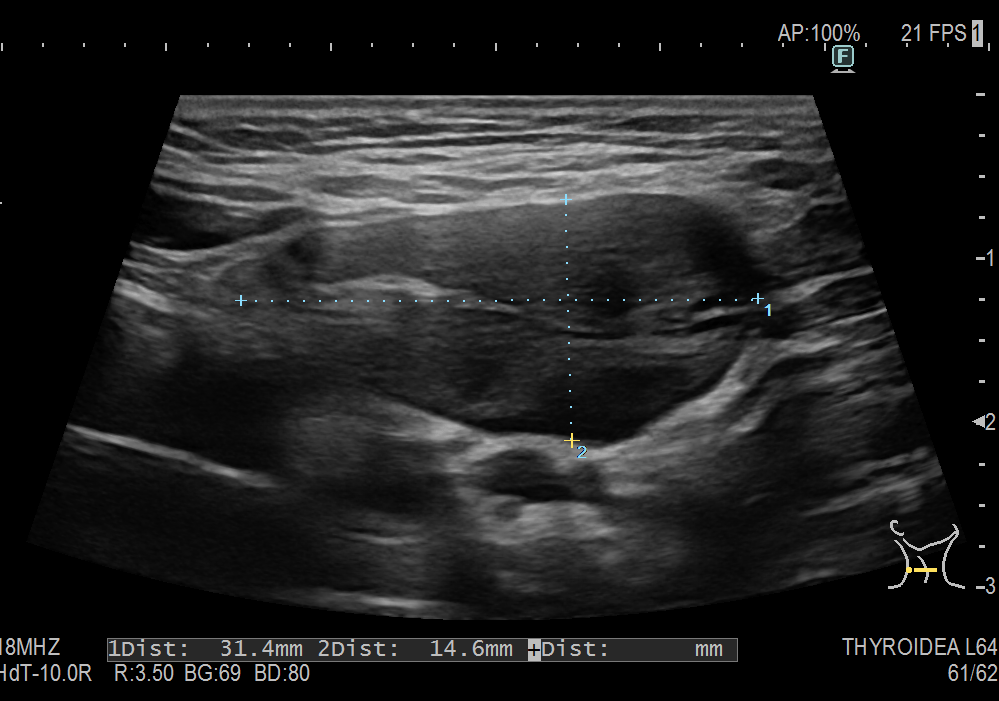
The general abdominal ultrasound examination revealed no abnormal findings transabdominally. This correlated with an external MRI examination, which was performed in view of the presence of an abdominal tumor. A focused ultrasound of the abdominal wall revealed a non-cystic mass in the middle of the rectus abdominis muscle with expansion. This was also consistent with an unclear change in the abdominal wall described in the MRI.
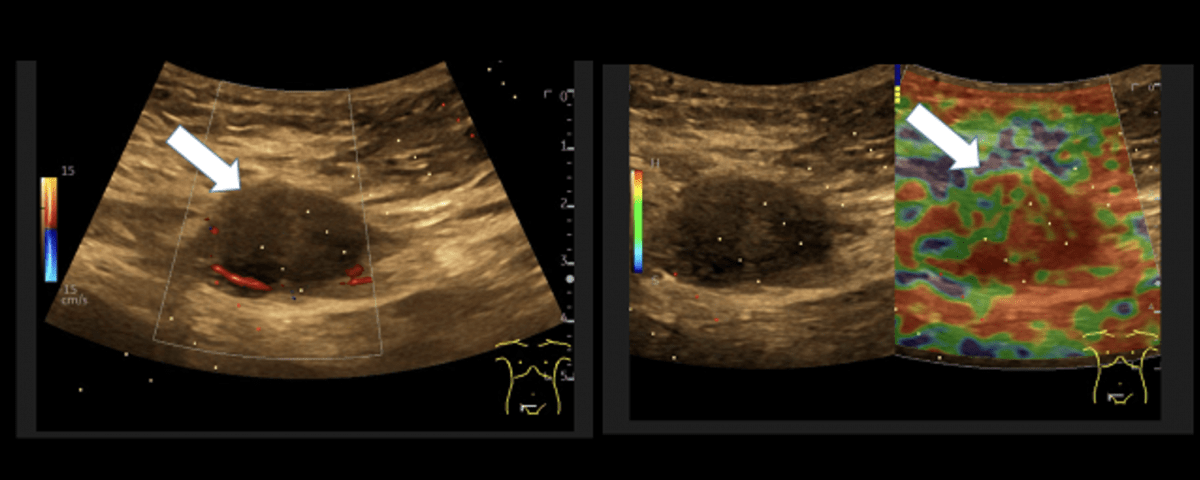
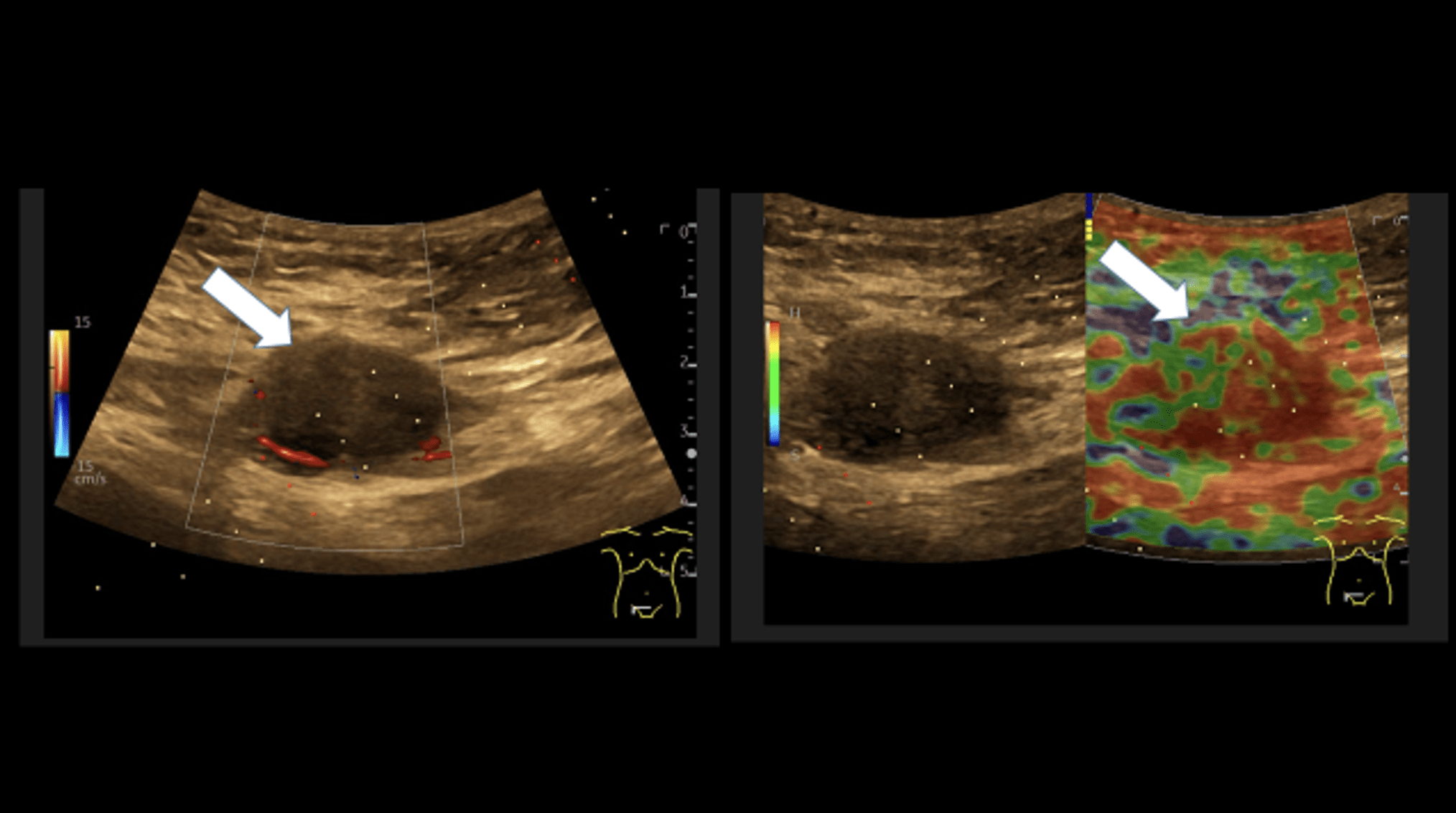
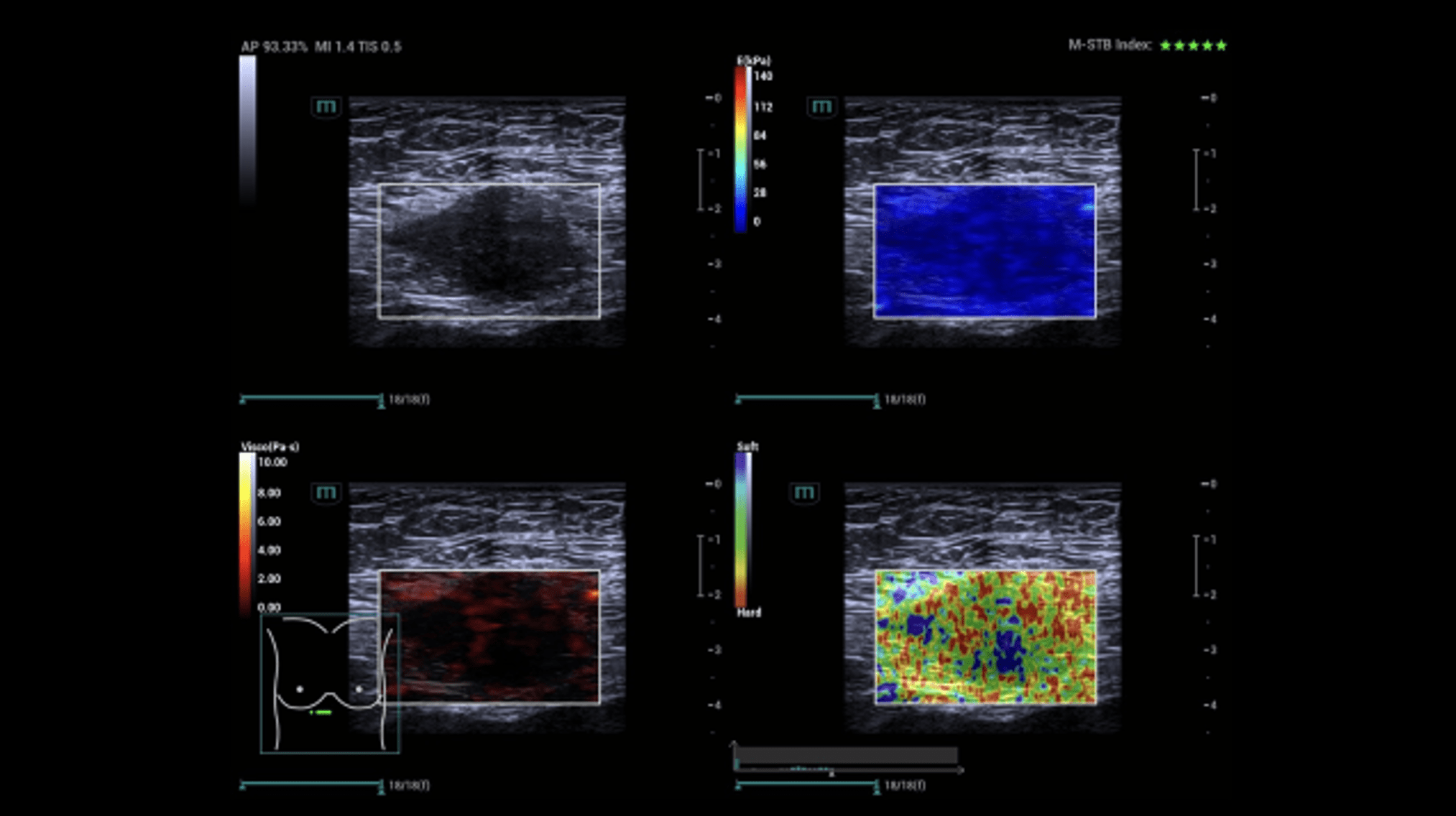
Sonography of the abdominal wall (Figures 3-6):
The ultrasound findings in the B-mode showed a proportion of solid areas with central necrosis. Color Doppler revealed marginal hypervascularization. Elastography revealed inhomogeneous tissue changes without nodular indurations typical of malignancy. Contrast-enhanced ultrasonography (CEUS) with perfusion evaluation after bolus administration of 2.4 ml SonoVue® i.v. showed evidence of increased peripheral capillary hypervascularization without malignancy-typical wash out.
Sonography-guided biopsy (Figure 7):
After obtaining written consent and allowing sufficient time for reflection, a targeted tissue biopsy of the mass was performed under local anesthesia under sterile conditions and ultrasound guidance. A biopsy-experienced examiner used a convex transducer with an appropriate biopsy device and a high-speed needle (14 G) to remove a 2 cm long tissue specimen from the mass in the lower abdomen in the area of the rectus abdominis muscle. The specimen fixed in formalin was considered representative for the histopathologic diagnosis and further examined.
The following differential diagnoses were made:
1. Inflammatory soft tissue reaction
2. Metastasis
3. Sarcoma
4. Muscle injury with reactive changes
5. Endometriosis with scar
Histopathology:
Macroscopy: White punched cylinder 2.0 cm long.
Microscopy: Sclerosed tissue structures of a fascia with partial atrophy of musculature and inclusion of endometrial islands loosely distributed within the partially scarred tissue and revealing hemosiderin-loaded macrophages. PAS reaction was inconspicuous.
The general abdominal ultrasound examination revealed no abnormal findings transabdominally. This correlated with an external MRI examination, which was performed in view of the presence of an abdominal tumor. A focused ultrasound of the abdominal wall revealed a non-cystic mass in the middle of the rectus abdominis muscle with expansion. This was also consistent with an unclear change in the abdominal wall described in the MRI.
Sonography of the abdominal wall (Figures 3-6):
The ultrasound findings in the B-mode showed a proportion of solid areas with central necrosis. Color Doppler revealed marginal hypervascularization. Elastography revealed inhomogeneous tissue changes without nodular indurations typical of malignancy. Contrast-enhanced ultrasonography (CEUS) with perfusion evaluation after bolus administration of 2.4 ml SonoVue® i.v. showed evidence of increased peripheral capillary hypervascularization without malignancy-typical wash out.
Sonography-guided biopsy (Figure 7):
After obtaining written consent and allowing sufficient time for reflection, a targeted tissue biopsy of the mass was performed under local anesthesia under sterile conditions and ultrasound guidance. A biopsy-experienced examiner used a convex transducer with an appropriate biopsy device and a high-speed needle (14 G) to remove a 2 cm long tissue specimen from the mass in the lower abdomen in the area of the rectus abdominis muscle. The specimen fixed in formalin was considered representative for the histopathologic diagnosis and further examined.
The following differential diagnoses were made:
1. Inflammatory soft tissue reaction
2. Metastasis
3. Sarcoma
4. Muscle injury with reactive changes
5. Endometriosis with scar
Histopathology:
Macroscopy: White punched cylinder 2.0 cm long.
Microscopy: Sclerosed tissue structures of a fascia with partial atrophy of musculature and inclusion of endometrial islands loosely distributed within the partially scarred tissue and revealing hemosiderin-loaded macrophages. PAS reaction was inconspicuous.
3Diagnosis
Nodular scarring fibrosis as a result of endometriosis of the abdominal wall.
4Discussion
BACKGROUND:
Despite its rather rare occurrence, intramuscular endometriosis is an important diagnosis in which multimodal ultrasound (US) can provide guidance and enable targeted histological confirmation. Depending on the localization, an endosonographic procedure (e.g. transvaginal ultrasound-TVUS) may also be necessary. With modern techniques such as elastography or CEUS including perfusion analysis, the differential diagnosis in the direction of malignant changes can be delineated more precisely.
Studies show that non-invasive imaging may have far-reaching implications for future research in endometriosis, as studies can now be performed without the need for prior surgery. Improving the diagnostic performance of US/TVUS alone or in combination with MRI is likely to occur over time and using powerful new technologies, such as AI. A main limitation at present is that there is a lack of personnel trained and experienced enough to perform and report sufficient US/TVUS for specific issues such as in the case described.
CLINICAL PERSPECTIVE:
While doctors who do not regularly work with ultrasound scanners have difficulty assessing an ultrasound image for the presence of endometriosis, the standard imaging planes and larger field of view of MRI imaging provide greater clarity, especially when the anatomy is highly distorted. However, MRI requires significantly more organization and is not always available. The use of ultrasound, on the other hand, is quickly available and can be carried out with less effort if the examiner has sufficient experience.
Overall, ultrasound and MRI imaging of endometriosis is a rapidly developing field that is being increasingly integrated into everyday clinical practice at a rapid pace.
THERAPY PLANNING:
Therapeutic options for endometriosis include both medical and surgical procedures. However, a cure is currently not possible.
OUTCOME & PROGNOSIS:
The case was presented and discussed on an interdisciplinary basis. The further therapeutic procedure is still pending.
Despite its rather rare occurrence, intramuscular endometriosis is an important diagnosis in which multimodal ultrasound (US) can provide guidance and enable targeted histological confirmation. Depending on the localization, an endosonographic procedure (e.g. transvaginal ultrasound-TVUS) may also be necessary. With modern techniques such as elastography or CEUS including perfusion analysis, the differential diagnosis in the direction of malignant changes can be delineated more precisely.
Studies show that non-invasive imaging may have far-reaching implications for future research in endometriosis, as studies can now be performed without the need for prior surgery. Improving the diagnostic performance of US/TVUS alone or in combination with MRI is likely to occur over time and using powerful new technologies, such as AI. A main limitation at present is that there is a lack of personnel trained and experienced enough to perform and report sufficient US/TVUS for specific issues such as in the case described.
CLINICAL PERSPECTIVE:
While doctors who do not regularly work with ultrasound scanners have difficulty assessing an ultrasound image for the presence of endometriosis, the standard imaging planes and larger field of view of MRI imaging provide greater clarity, especially when the anatomy is highly distorted. However, MRI requires significantly more organization and is not always available. The use of ultrasound, on the other hand, is quickly available and can be carried out with less effort if the examiner has sufficient experience.
Overall, ultrasound and MRI imaging of endometriosis is a rapidly developing field that is being increasingly integrated into everyday clinical practice at a rapid pace.
THERAPY PLANNING:
Therapeutic options for endometriosis include both medical and surgical procedures. However, a cure is currently not possible.
OUTCOME & PROGNOSIS:
The case was presented and discussed on an interdisciplinary basis. The further therapeutic procedure is still pending.
5Teaching Points
Modern ultrasound techniques, including biopsy procedures, can be a useful, readily available and effective method to improve the sectional imaging based diagnosis of endometriosis. However, multimodal ultrasound experience is a clear prerequisite.
6References
1.) Bulun SE, Yilmaz BD, Sison C et al. Endometriosis. Endocr Rev. 2019 ;40(4):1048-1079.
2.) Mariuzzi L, Domenis R, Orsaria M et al. Functional expression of aryl hydrocarbon receptor on mast cells populating human endometriotic tissues. Lab Invest. 2016; 96(9): 959-971.
3.) Xholli A, Filip G, Previtera F et al. Modification of endometrioma size during hormone therapy containing dienogest. Gynecol Endocrinol. 2020 ; 36(6): 545-549.
4.) Avery JC , Deslandes A, Freger SM et al. Noninvasive diagnostic imaging for endometriosis part 1: a systematic review of recent developments in ultrasound, combination imaging, and artificial intelligence. Fertil Steril. 2024; 121(2): 164-188.
5.) Jung EM, Kaiser U, Herr W et al. Novel high-resolution contrast agent ultrasound techniques HiFR CEUS and SR CEUS in combination with shear wave elastography, fat assessment and viscosity of liver parenchymal changes and tumors. Clin Hemorheol Microcirc. 2024. DOI: 10.3233/CH-249103. Online ahead of print.
6.) Xholli A , Londero AP , Cavalli E et al. The benefit of transvaginal elastography in detecting deep endometriosis: a feasibility study. Ultraschall Med 2024; 45(01): 69-76.
7.) Ding D, Chen Y, LiuX et al. Diagnosing deep endometriosis using transvaginal elastosonography. Reprod Sci. 2020; 27(7): 1411-1422.
2.) Mariuzzi L, Domenis R, Orsaria M et al. Functional expression of aryl hydrocarbon receptor on mast cells populating human endometriotic tissues. Lab Invest. 2016; 96(9): 959-971.
3.) Xholli A, Filip G, Previtera F et al. Modification of endometrioma size during hormone therapy containing dienogest. Gynecol Endocrinol. 2020 ; 36(6): 545-549.
4.) Avery JC , Deslandes A, Freger SM et al. Noninvasive diagnostic imaging for endometriosis part 1: a systematic review of recent developments in ultrasound, combination imaging, and artificial intelligence. Fertil Steril. 2024; 121(2): 164-188.
5.) Jung EM, Kaiser U, Herr W et al. Novel high-resolution contrast agent ultrasound techniques HiFR CEUS and SR CEUS in combination with shear wave elastography, fat assessment and viscosity of liver parenchymal changes and tumors. Clin Hemorheol Microcirc. 2024. DOI: 10.3233/CH-249103. Online ahead of print.
6.) Xholli A , Londero AP , Cavalli E et al. The benefit of transvaginal elastography in detecting deep endometriosis: a feasibility study. Ultraschall Med 2024; 45(01): 69-76.
7.) Ding D, Chen Y, LiuX et al. Diagnosing deep endometriosis using transvaginal elastosonography. Reprod Sci. 2020; 27(7): 1411-1422.