- European Federation of Societies for Ultrasound in Medicine and Biology ~ Educating all for competence to practice ultrasound safely
CEUS Beyond Europe – Chinese Experience
September 19, 2016
One-stop differential diagnosis of Mllerian anomalies using 3D ultrasound: complete uterine septum [Nov 2016]
November 11, 2016Abdominal (retroperitoneal) lymphadenopathy
Correspondence: Prof. Dr. med. Christoph F. Dietrich
Medizinische Klinik 2, Caritas-Krankenhaus
Uhlandstr. 7. 97980 Bad Mergentheim
Tel:+49 7931 58 2201
Email: christoph.dietrich@ckbm.de
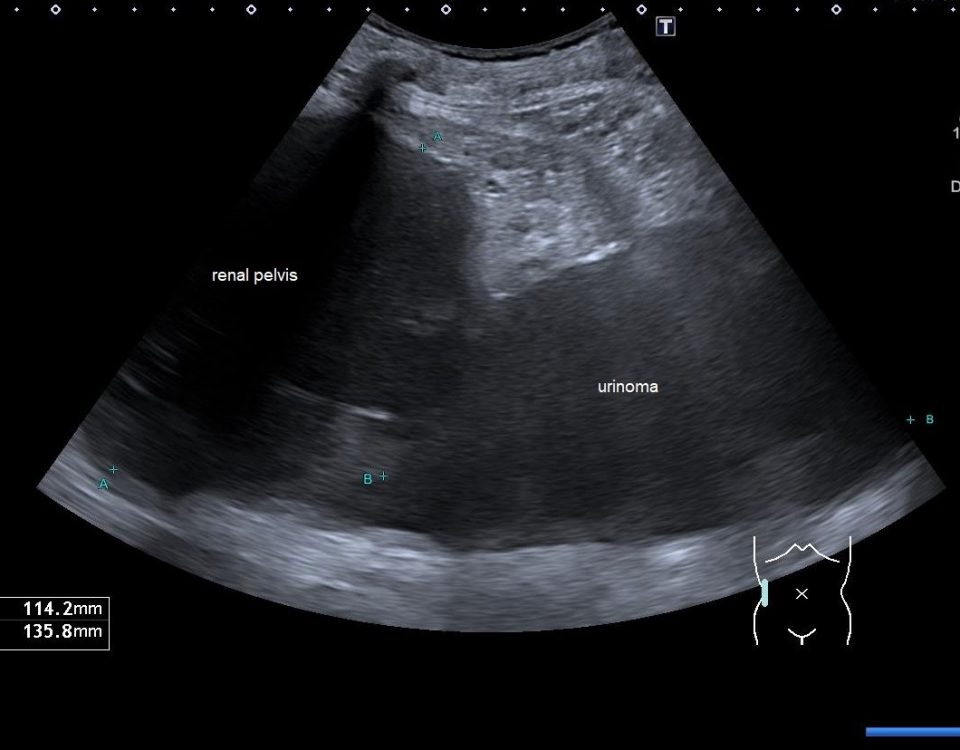
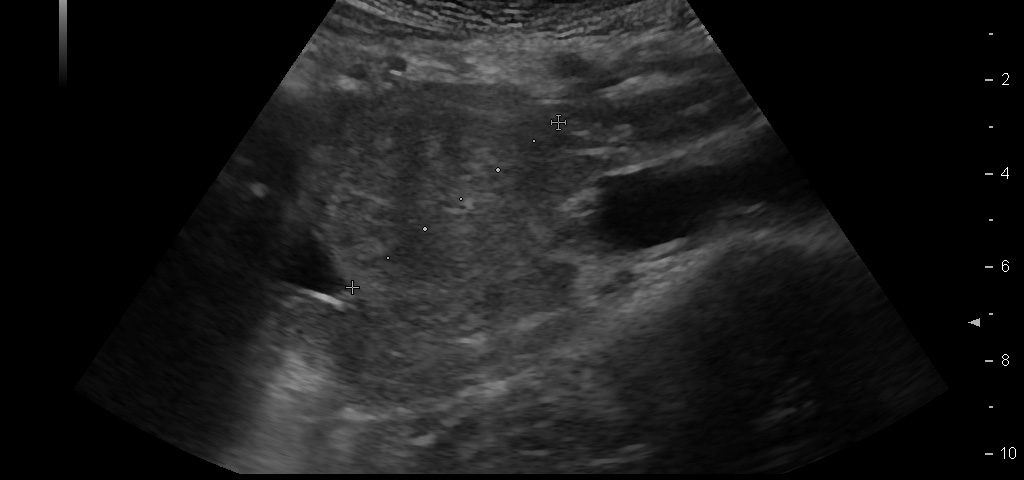
We report on a 28 year old male presenting with unspecific abdominal pain for two weeks. B-mode ultrasound revealed extended abdominal lymphadenopathy [Figure 1] and two small unspectacular hemangioma-like focal liver lesions [Figure 2]. The left testis revealed a palpable tumour by ultrasound also examined with elastography and contrast enhanced ultrasound [Figure 3 and 4]. Endoscopy of the upper gastrointestinal tract showed candida of the esophagus. The biopsy of the abdominal (retroperitoneal) lymph nodes revealed epithelial cells, typical for testicular embryonal cell carcinoma.
This case report illustrates the use of conventional and innovative ultrasound technologies for the differential diagnosis of (retroperitoneal) lymphadenopathy [(6-18)], focal liver lesions [(2;19-24)], and testicular masses [(25-32)] in daily routine [(33)]. The following text mainly refers to the published paper in Endoskopie Heute [(34)]. Testicular tumours account for <2 % of all malignancies in men and are thus considered rare. There is a marked age association with 20 - 40 year old patients (mean age 35) accounting for 20 - 30 % of all cases, and testicular tumours are the most common malignant tumour in this age group. The classic initial symptom in all testicular cancers is the painless, unilateral and slow increase in size of the testis but sometimes also unspecific symptoms elsewhere. Testicular tumours can be divided into germ cell and non-germ cell tumours. The most common histological type of germ cell tumour (and ultimately of all testicular tumours, 40%) is the seminoma. The seminoma appears sonographically as a hypoechoic, relatively sharply defined tumour without calcification or cystic structures. In our patient small calcifications could be seen. Non-seminomatous germ cell tumours include embryonal carcinomas (as in our case) teratocarcinomas, teratomas, choriocarcinoma, and yolk sac tumours. Mixed tumours are rare and show a variety of elastography appearances. Other tumours of the gonadal stroma, metastases and lymphomas have to be ruled out before surgery. Sonographic appearances of the Leydig cell tumour (2 % of all testicular tumours) cannot be differentiated from those of seminoma. The echo pattern is similar to seminomas, the Leydig cell tumour is usually smaller. Differential diagnosis has to take into account haematoma, and focal changes in granulomatous orchitis (anamnestic information). According to testicular imaging we also refer to the already mentioned published literature on imaging [(25-32)]. Other pathological entities include testicular cysts and (micro) calcifications. Microlithiasis is often observed in combination with a malignant testicular tumour in the surrounding parenchyma [(26)]. The value of elastography for early detection and avoidance of biopsy procedures has not been established so far but is promising [(34)]. Lymphatic drainage of the testis is ipsilateral, lateral to the para-aortic lymph nodes, located at the level of the renal vessels on the left, and slightly more caudally on the right. The case illustrates lymph node assessment criteria. For further reading we refer to the recently published literature [(7-9;14;35-37)]. Regarding CEUS of the liver [(24;38;39)], CEUS of non-liver organs [(39)], elastography [(40-44)], and ultrasound guided interventions [(45-53)] we refer to the EFSUMB guidelines [(54)].
- Dietrich CF, Mertens JC, Braden B, Schuessler G, Ott M, Ignee A. Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology 2007; 45(5):1139-1145.
- Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med 2012; 33(4):344-351.
- Frohlich E, Muller R, Cui XW, Schreiber-Dietrich D, Dietrich CF. Dynamic contrast-enhanced ultrasound for quantification of tissue perfusion. J Ultrasound Med 2015; 34(2):179-196.
- Ignee A, Jedrejczyk M, Schuessler G, Jakubowski W, Dietrich CF. Quantitative contrast enhanced ultrasound of the liver for time intensity curves-Reliability and potential sources of errors. Eur J Radiol 2010; 73(1):153-158.
- Cui XW, Ignee A, Jedrzejczyk M, Dietrich CF. Dynamic Vascular Pattern (DVP), a quantification tool for contrast enhanced ultrasound. Z Gastroenterol 2013; 51(5):427-431.
- Chiorean L, Barr RG, Braden B, Jenssen C, Cui XW, Hocke M et al. Transcutaneous Ultrasound: Elastographic Lymph Node Evaluation. Current Clinical Applications and Literature Review. Ultrasound Med Biol 2016; 42(1):16-30.
- Cui XW, Jenssen C, Saftoiu A, Ignee A, Dietrich CF. New ultrasound techniques for lymph node evaluation. World J Gastroenterol 2013; 19(30):4850-4860.
- Cui XW, Ignee A, Bachmann NM, Schreiber-Dietrich D, De Molo C, Pirri C et al. Contrast enhanced ultrasound of sentinel lymph nodes. J Ultrason 2013; 13:73-81.
- Cui XW, Hocke M, Jenssen C, Ignee A, Klein S, Schreiber-Dietrich D et al. Conventional ultrasound for lymph node evaluation, update 2013. Z Gastroenterol 2014; 52(2):212-221.
- Barreiros AP, Braden B, Schieferstein-Knauer C, Ignee A, Dietrich CF. Characteristics of intestinal tuberculosis in ultrasonographic techniques. Scand J Gastroenterol 2008; 43(10):1224-1231.
- Braden B, Faust D, Ignee A, Schreiber D, Hirche T, Dietrich CF. Clinical relevance of perihepatic lymphadenopathy in acute and chronic liver disease. J Clin Gastroenterol 2008; 42(8):931-936.
- Dietrich CF, Viel K, Braden B, Caspary WF, Zeuzem S. [Mediastinal lymphadenopathy: an extrahepatic manifestation of chronic hepatitis C?]. Z Gastroenterol 2000; 38(2):143-152.
- Dietrich CF, Leuschner MS, Zeuzem S, Herrmann G, Sarrazin C, Caspary WF et al. Peri-hepatic lymphadenopathy in primary biliary cirrhosis reflects progression of the disease. Eur J Gastroenterol Hepatol 1999; 11(7):747-753.
- Dietrich CF, Hocke M, Jenssen C. [Ultrasound for abdominal lymphadenopathy]. Dtsch Med Wochenschr 2013; 138(19):1001-1018.
- Hirche TO, Russler J, Braden B, Schuessler G, Zeuzem S, Wehrmann T et al. Sonographic detection of perihepatic lymphadenopathy is an indicator for primary sclerosing cholangitis in patients with inflammatory bowel disease. Int J Colorectal Dis 2004; 19(6):586-594.
- Hirche TO, Hirche H, Cui XW, Wagner TO, Dietrich CF. Ultrasound evaluation of mediastinal lymphadenopathy in patients with sarcoidosis. Med Ultrason 2014; 16(3):194-200.
- Jenssen C, Annema JT, Clementsen P, Cui XW, Borst MM, Dietrich CF. Ultrasound techniques in the evaluation of the mediastinum, part 2: mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography. J Thorac Dis 2015; 7(10):E439-E458.
- Schreiber-Dietrich D, Pohl M, Cui XW, Braden B, Dietrich CF, Chiorean L. Perihepatic lymphadenopathy in children with chronic viral hepatitis. J Ultrason 2015; 15(61):137-150.
- Dietrich CF, Jenssen C. [Focal liver lesion, incidental finding]. Dtsch Med Wochenschr 2012; 137(41):2099-2116.
- Strobel D, Bernatik T, Blank W, Schuler A, Greis C, Dietrich CF et al. Diagnostic accuracy of CEUS in the differential diagnosis of small (
- Strobel D, Seitz K, Blank W, Schuler A, Dietrich CF, von Herbay A et al. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS). Ultraschall Med 2009; 30(4):376-382.
- Dietrich CF, Cui XW, Barreiros AP, Hocke M, Ignee A. EFSUMB guidelines 2011: comment on emergent indications and visions. Ultraschall Med 2012; 33 Suppl 1:S39-S47.
- Dietrich CF, Cui XW, Schreiber-Dietrich DG, Ignee A. EFSUMB guidelines 2011: comments and illustrations. Ultraschall Med 2012; 33 Suppl 1:S11-S21.
- Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013; 39(2):187-210.
- Bertolotto M, Derchi LE, Secil M, Dogra V, Sidhu PS, Clements R et al. Grayscale and color Doppler features of testicular lymphoma. J Ultrasound Med 2015; 34(6):1139-1145.
- O'Flynn EA, Sidhu PS. The sonographic twinkling artifact in testicular calcification. J Ultrasound Med 2009; 28(4):515-517.
- Patel KV, Navaratne S, Bartlett E, Clarke JL, Muir GH, Sellars ME et al. Testicular Microlithiasis: Is Sonographic Surveillance Necessary? Single Centre 14 Year Experience in 442 Patients with Testicular Microlithiasis. Ultraschall Med 2015.
- Patel KV, Huang DY, Sidhu PS. Metachronous bilateral segmental testicular infarction: multi-parametric ultrasound imaging with grey-scale ultrasound, Doppler ultrasound, contrast-enhanced ultrasound (CEUS) and real-time tissue elastography (RTE). J Ultrasound 2014; 17(3):233-238.
- Sellars ME, Sidhu PS. Ultrasound appearances of the testicular appendages: pictorial review. Eur Radiol 2003; 13(1):127-135.
- Sidhu PS, Sriprasad S, Bushby LH, Sellars ME, Muir GH. Impalpable testis cancer. BJU Int 2004; 93(6):888.
- Stewart VR, Sidhu PS. The testis: the unusual, the rare and the bizarre. Clin Radiol 2007; 62(4):289-302.
- Yusuf G, Konstantatou E, Sellars ME, Huang DY, Sidhu PS. Multiparametric Sonography of Testicular Hematomas: Features on Grayscale, Color Doppler, and Contrast-Enhanced Sonography and Strain Elastography. J Ultrasound Med 2015; 34(7):1319-1328.
- Frohlich E, Jenssen C, Schuler A, Dietrich CF. [Contrast-enhanced ultrasound for characterisation of focal liver lesions, practical advice]. Z Gastroenterol 2015; 53(9):1099-1107.
- Dietrich CF. Real-time tissue elastography. Multiple clinical applications. Multiple clinical solutions. Endoskopie heute 2012; 24:177-212.
- Cui XW, Chang JM, Kan QC, Chiorean L, Ignee A, Dietrich CF. Endoscopic ultrasound elastography: Current status and future perspectives. World J Gastroenterol 2015; 21(47):13212-13224.
- Dietrich CF, Zeuzem S, Caspary WF, Wehrmann T. [Ultrasound lymph node imaging in the abdomen and retroperitoneum of healthy probands]. Ultraschall Med 1998; 19(6):265-269.
- Dietrich CF, Ponnudurai R, Bachmann NM. [Is there a need for new imaging methods for lymph node evaluation?]. Ultraschall Med 2012; 33(5):411-414.
- Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med 2008; 29(1):28-44.
- Piscaglia F, Nolsoe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann NM et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012; 33(1):33-59.
- Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 2013; 34(2):169-184.
- Barr RG, Nakashima K, Amy D, Cosgrove D, Farrokh A, Schafer F et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 2: Breast. Ultrasound Med Biol 2015; 41(5):1148-1160.
- Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography.Part 2: Clinical Applications. Ultraschall Med 2013; 34(3):238-253.
- Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 3: Liver. Ultrasound Med Biol 2015; 41(5):1161-1179.
- Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic Principles and Terminology. Ultrasound Med Biol 2015; 41(5):1126-1147.
- Lorentzen T, Nolsoe CP, Ewertsen C, Nielsen MB, Leen E, Havre RF et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part I. General Aspects (Short Version). Ultraschall Med 2015; 36(5):464-472.
- Lorentzen T, Nolsoe CP, Ewertsen C, Nielsen MB, Leen E, Havre RF et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part I. General Aspects (long Version). Ultraschall Med 2015; 36(5):E1-14.
- Sidhu PS, Brabrand K, Cantisani V, Correas JM, Cui XW, D'Onofrio M et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part II. Ultraschall Med 2015; 36(6):566-580.
- Sidhu PS, Brabrand K, Cantisani V, Correas JM, Cui XW, D'Onofrio M et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part II. Ultraschall Med 2015; 36(6):E15-E35.
- Fusaroli P, Jenssen C, Hocke M, Burmester E, Buscarini E, Havre RF et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part V. Ultraschall Med 2015.
- Dietrich CF, Lorentzen T, Appelbaum L, Buscarini E, Cantisani V, Correas JM et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III. Ultraschall Med 2015.
- Dietrich CF. EFSUMB guidelines 2015 on interventional ultrasound. Med Ultrason 2015; 17(4):521-527.
- Dietrich CF, Lorentzen T, Sidhu PS, Jenssen C, Gilja OH, Piscaglia F. An Introduction to the EFSUMB Guidelines on Interventional Ultrasound (INVUS). Ultraschall Med 2015; 36(5):460-463.
- Jenssen C, Brkljacic B, Hocke M, Ignee A, Piscaglia F, Radjina M et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part VI - Ultrasound-Guided Vascular Interventions. Ultraschall Med 2015.
- Dietrich CF, Rudd L. The EFSUMB website, a guide for better understanding. Med Ultrason 2013; 15(3):215-223.
Figure 2: Focal liver lesion using B-mode (a) and contrast enhanced ultrasound (CEUS) in the arterial (b), late phase (c) and CEUS using time intensity curve analysis (TICA) (d). The B-mode ultrasound showed an isoechoic lesion with transducer distal shadowing, somewhat unspectacular. CEUS showed early and slightly hyperenhancing features in the arterial phase and pronounced wash out in the portal venous and late phases indicating metastases and excluding hemangioma [(1)]. The TICA image on the right side of the screen differentiates the initially hyperenhancing lesion (red line) in comparison to the surrounding liver parenchyma (yellow line). In the portal venous phase the red line crosses downward in comparison to the liver parenchyma (yellow line), indicating metastasis [(2-5)].
Figure 3: Imaging of the left testicle using elastography with sharp delineation of the embryonal cell carcinoma with clear stiffer elastographic delineation from the surrounding tissue (a) and CEUS with atypical neoplastic vessels and little perfusion (b).


![Abdominal (retroperitoneal) lymphadenopathy</br> [Oct 2016]](https://efsumb.org/wp-content/uploads/2020/11/cotm_october2016_fg1a.jpg)
![Abdominal (retroperitoneal) lymphadenopathy</br> [Oct 2016]](https://efsumb.org/wp-content/uploads/2020/11/cotm_october2016_fg1b.jpg)
![Abdominal (retroperitoneal) lymphadenopathy</br> [Oct 2016]](https://efsumb.org/wp-content/uploads/2020/11/cotm_october2016_fg2a.jpg)
![Abdominal (retroperitoneal) lymphadenopathy</br> [Oct 2016]](https://efsumb.org/wp-content/uploads/2020/11/cotm_october2016_fg2b.jpg)
![Abdominal (retroperitoneal) lymphadenopathy</br> [Oct 2016]](https://efsumb.org/wp-content/uploads/2020/11/cotm_october2016_fg2c.jpg)
![Abdominal (retroperitoneal) lymphadenopathy</br> [Oct 2016]](https://efsumb.org/wp-content/uploads/2020/11/cotm_october2016_fg2d.jpg)
![Abdominal (retroperitoneal) lymphadenopathy</br> [Oct 2016]](https://efsumb.org/wp-content/uploads/2020/11/cotm_october2016_fg3a.jpg)
![Abdominal (retroperitoneal) lymphadenopathy</br> [Oct 2016]](https://efsumb.org/wp-content/uploads/2020/11/cotm_october2016_fg3b.jpg)