Diverticula and diverticulitis of the appendix [Jul 2017]
July 11, 2017Liver Elastography
September 7, 2017Gastric GIST coexisting with primary lung adenocarcinoma
1 Clinic of Gastroenterology, 2 Department of Pathology, 3Department of Radiology, University Hospital “Tsaritsa Yoanna – ISUL”, Medical University Sofia, Bulgaria.
A rare case of pulmonary adenocarcinoma and synchronous large gastric GIST, was diagnosed with US, EUS, CEUS and CECT. Percutaneous true-cut biopsy of GIST ensured immunohistochemical evaluation. The patient was treated and followed up for 1 year.
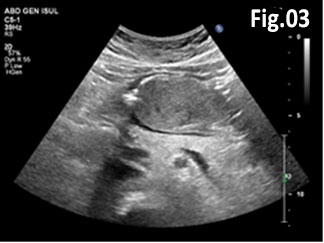
GISTs are rare mesenchymal neoplasms of the digestive tract [1,2]. They have been documented in all parts of the gastrointestinal tract, but are most common in the stomach and small intestine, followed by colo-rectum, mesentery, and esophagus [1,2]. However, the synchronous occurrence of lung cancer and GIST is extremely rare [3]. The incidence of GIST coexisting with additional malignancies is 9% to 27%, and primary lung cancer in GIST patients is 0.5%-1.2% [3]. A 75 year old woman with a smoking history for >30 years was diagnosed with advanced stage of left lung primary adenocarcinoma by chest computed tomography (CT) and bronchoscopy with sampling in February 2015 (Fig.1, Fig.2). The patient started Erlotinib 150 mg/day. In September 2015 the patient was admitted with vague abdominal pain and postprandial vomiting. Physical examination, routine blood, urine and stool investigations were unremarkable. Abdominal ultrasound detected an oval, homogenous, hypoechoic lesion 6/5/4 cm, arising from the submucosal layers of the gastric wall (Fig 3). The color Doppler demonstrated neovascularization (Fig 4). Except for 3 small simple liver cysts, no other abnormalities were present. Upper endoscopy and endoscopic ultrasound (EUS) revealed a large oval, slightly lobulated mass, hypoechoic 6x5cm between incisura angulars and greater curvature of the gastric body, originating from the 4th layer of the wall (muscularis propria). The tumor was slightly “dumbbell” shaped, protruding in and outside the stomach lumen, with several irregular hypoechoic zones and micro cysts within, looked encapsulated without infiltration of the surrounding tissues and pathologic abdominal lymph nodes (Fig. 5). A contrast-enhanced ulstrasound (CEUS) and contrast-enhanced computed tomography (CECT) of the abdomen additionally characterized the lesion and excluded liver metastases. (Fig. 6, Fig. 7) Percutaneous true-cut biopsy of the lesion was performed (18G, two separate puncture sites) (Fig 8). The immunohistochemical analysis revealed epithelioid-type GIST, diffusely positive for c-kit (CD117), with a weak focal expression of S-100 (Fig. 9). Due to life expectancy defined by the unresectable advanced lung cancer, the patient was not indicated for resection of the gastric GIST. Imatinib (Glivec) 400 mg/day was started as a “co-therapy” to Erlotinib in November 2015. After 12 months of co-treatment, a good control of both tumors was achieved. CT and abdominal ultrasound showed the gastric lesion was slightly shrunk on therapy, with large central necrosis (Fig. 10). The patient is being followed up.
Clinic of Gastroenterology
University Hospital “Tsaritsa Yoanna – ISUL”, Medical University Sofia.
Byalo more str. № 8, Sofia 1527, Bulgaria. Tel: +359878813841
Email: ivan.lutakov@gmail.com
- 1 Pidhorecky I, Cheney RT, Kraybill WG et al. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol 2000; 7(9): 705–712.
- 2 Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006; 130(10): 1466–1478.
- 3 Wada Y, Koizumi T, Yokoyama T et al. Synchronous gastrointestinal stromal tumor and primary lung adenocarcinoma. Intern Med 2012; 51(17): 2407–2410.
Figure 2: Histology of the pulmonary adenocarcinoma
Figure 3: Abdominal ultrasound: a solid hypoechoic oval tumor arising from the gastric wall
Figure 4: Color Doppler: Tumor neovascularization
Figure 5: EUS of the gastric tumor: “dumbbell” shape, irregular hypoechoic zones and microcysts, high-risk GIST suspected.
Figure 6. CEUS: Arterial hyperenhancement and early venous wash-out
Figure 7. CT(CECT): Hyperenhanced tumor with heterogeneous structure, sized 68х57x45 mm, probably GIST. No significant lymph nodes or secondary lesions. Small simple liver cysts.
Figure 8: A true-cut biopsy of the lesion - 18G, two separate puncture sites.
Figure 9. Epithelioid-type GIST. Immunohistochemical analysis: CD117/+++/, S-100/+/.
Figure 10: CT and US at 12 month follow-up: Reduced lesion size and central necrosis.


![Gastric GIST coexisting with primary lung adenocarcinoma</br> [Aug 2017]](https://efsumb.org/wp-content/uploads/2020/11/cotm_august2017_fig1n2.jpg)
![Gastric GIST coexisting with primary lung adenocarcinoma</br> [Aug 2017]](https://efsumb.org/wp-content/uploads/2020/11/cotm_august2017_fig3n4.jpg)
![Gastric GIST coexisting with primary lung adenocarcinoma</br> [Aug 2017]](https://efsumb.org/wp-content/uploads/2020/11/cotm_august2017_fig5.jpg)
![Gastric GIST coexisting with primary lung adenocarcinoma</br> [Aug 2017]](https://efsumb.org/wp-content/uploads/2020/11/cotm_august2017_fig6.jpg)
![Gastric GIST coexisting with primary lung adenocarcinoma</br> [Aug 2017]](https://efsumb.org/wp-content/uploads/2020/11/cotm_august2017_fig7.jpg)
![Gastric GIST coexisting with primary lung adenocarcinoma</br> [Aug 2017]](https://efsumb.org/wp-content/uploads/2020/11/cotm_august2017_fig8.jpg)
![Gastric GIST coexisting with primary lung adenocarcinoma</br> [Aug 2017]](https://efsumb.org/wp-content/uploads/2020/11/cotm_august2017_fig9.jpg)
![Gastric GIST coexisting with primary lung adenocarcinoma</br> [Aug 2017]](https://efsumb.org/wp-content/uploads/2020/11/cotm_august2017_fig10.jpg)