Student Image Challenge 106
May 16, 2024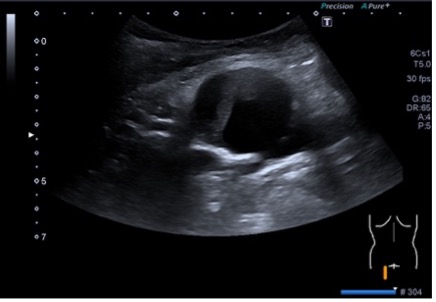
Student Image Challenge 107
June 13, 2024SUBMIT YOUR CASE
EFSUMB invites submission of interesting cases for the website section 'Case of the Month'. All CoM submissions are eligible for selection for free registration at the next Euroson congress. Two cases that receive the most 'likes' in a year will receive free registration for the next EUROSON congress and the third most liked liked case will receive a cash prize of 100 EUR.
Microbubble leakage into the intestine
Authors: Kaiser Ulrich[1], Daniel Wolff[1], Jung Ernst Michael[2]
[1] Medical Clinic and Polyclinic III, University Hospital Regensburg, Regensburg, Germany
[2] Institute for Diagnostic Radiology and Interdisciplinary Ultrasound, University Hospital Regensburg, Regensburg, Germany
1Clinical History
Various complications relating to the gastrointestinal tract can arise following allogeneic stem cell transplantation. In these cases, ultrasound diagnostics has proven to be an diagnostic tool for assessment of potential differential diagnoses.
This was also the case in the present case of a 56-year-old patient who presented to the ultrasound outpatient clinic with increasing pain in the lower abdomen and diarrhea following an allogeneic stem cell transplant performed 6 weeks previously.
This was also the case in the present case of a 56-year-old patient who presented to the ultrasound outpatient clinic with increasing pain in the lower abdomen and diarrhea following an allogeneic stem cell transplant performed 6 weeks previously.
2Image findings
ABDOMINAL SONOGRAPHY:
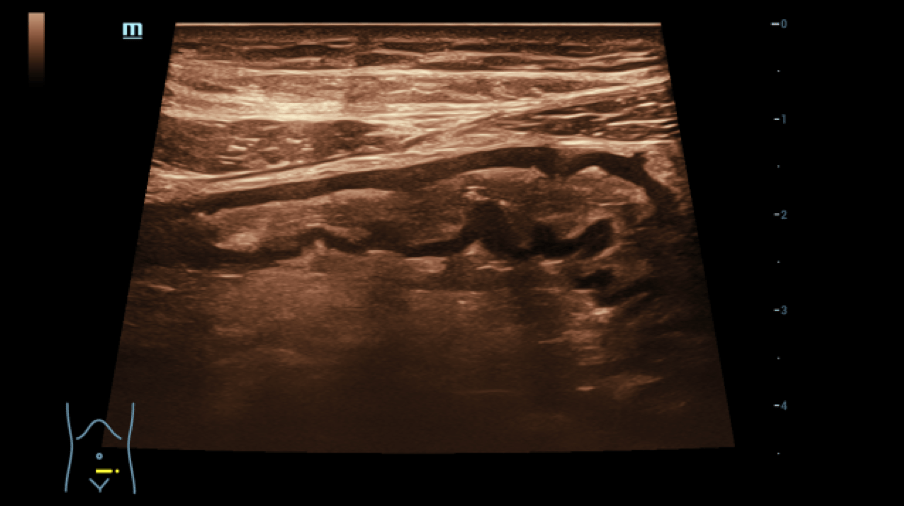
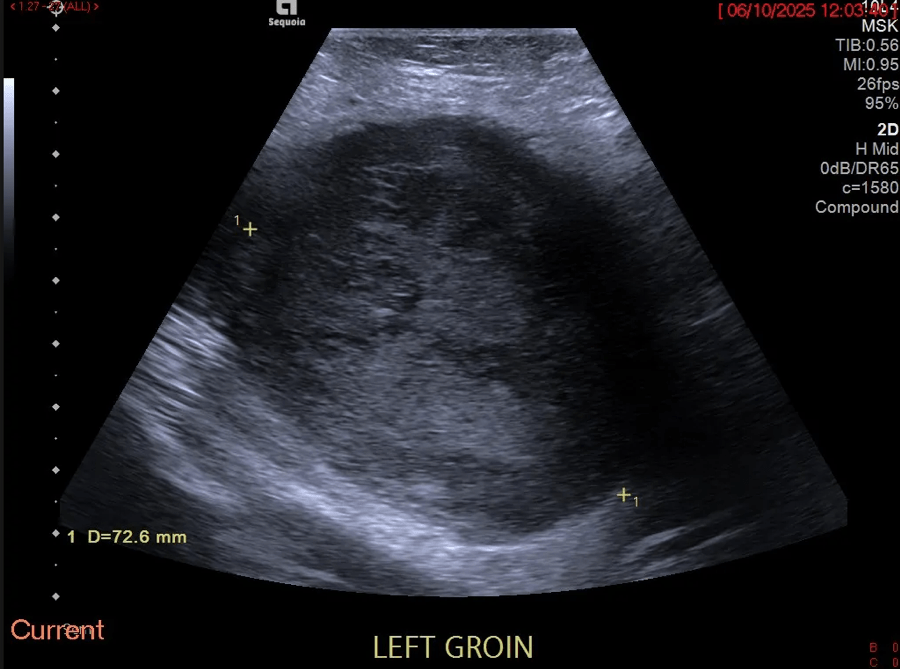
No free fluid was found in the FAST sonography. The abdominal sonography was performed with a convex multifrequency probe (C1-6 MHz) and the detailed assessment of the intestinal tract was additionally performed with a linear multifrequency probe (L6-18 MHz). This revealed an echo increase of the liver tissue in the sense of steatosis without cholestasis with unremarkable blood flow in the CCDS, the size of the spleen was 12x5 cm, there was no urinary retention with slight echo enlargement of a slightly narrowed renal parenchyma, no pathologically enlarged lymph nodes were detectable, the pancreas and gallbladder were not inflammatory. The stomach wall with 6 mm was slightly thickened in the area of the pylorus. The large intestine was clearly distended, which made the examination more difficult. The most striking finding was the ileum part of the small intestine.
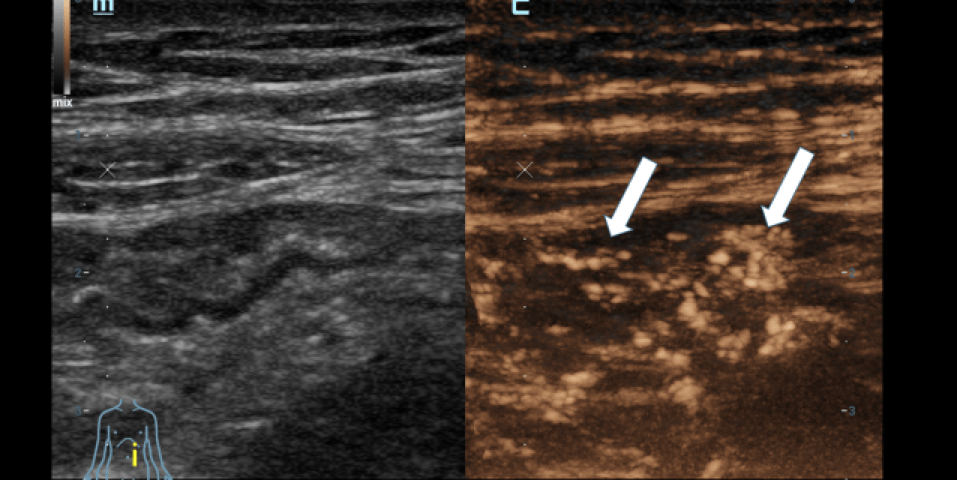
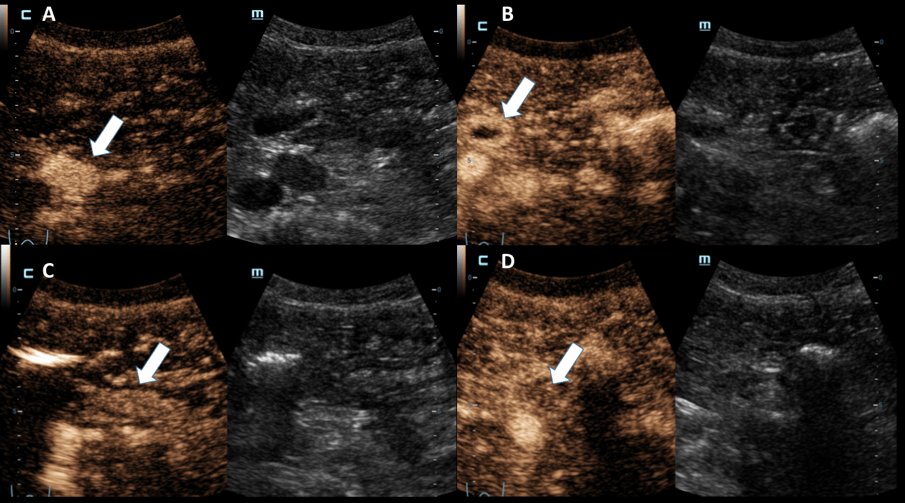
ULTRASOUND FINDINGS OF THE SMALL INTESTINE (Figures 1.2):
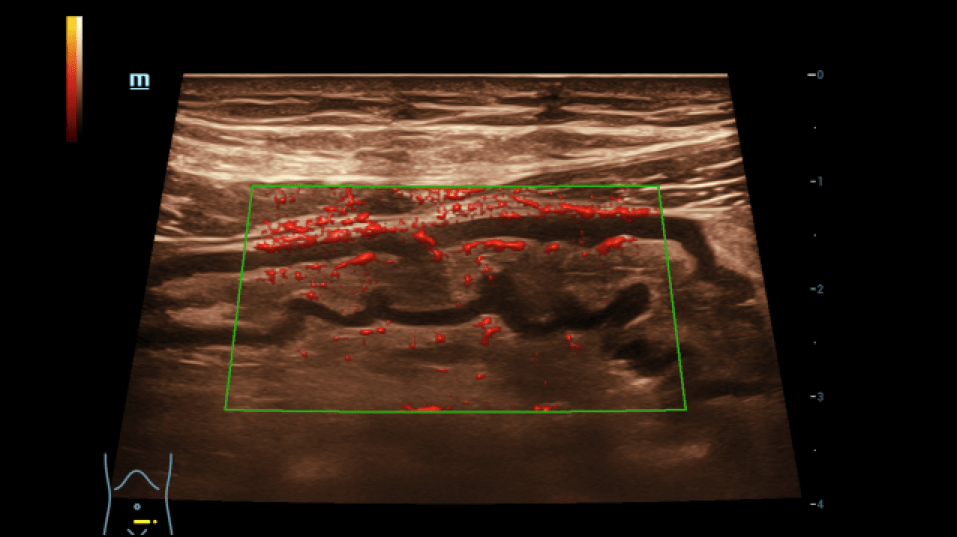
Particularly in the left middle abdomen, hyperemia with hypercirculation was found in the presence of intestinal wall edema (up to 8 mm) using CCDS. These reactive changes were detectable up to the preterminal ileum. Only slight hypercirculation was detectable mesenteric area. There was no extraluminal fluid content and no retention. The examination revealed no evidence of appendicitis.
This resulted in the following differential diagnoses:
- Bacterial enteritis
- Autoimmune reaction
- Drug-induced colitis
- Viral enteritis
- GvHD (Graft-versus-Host Reaction)
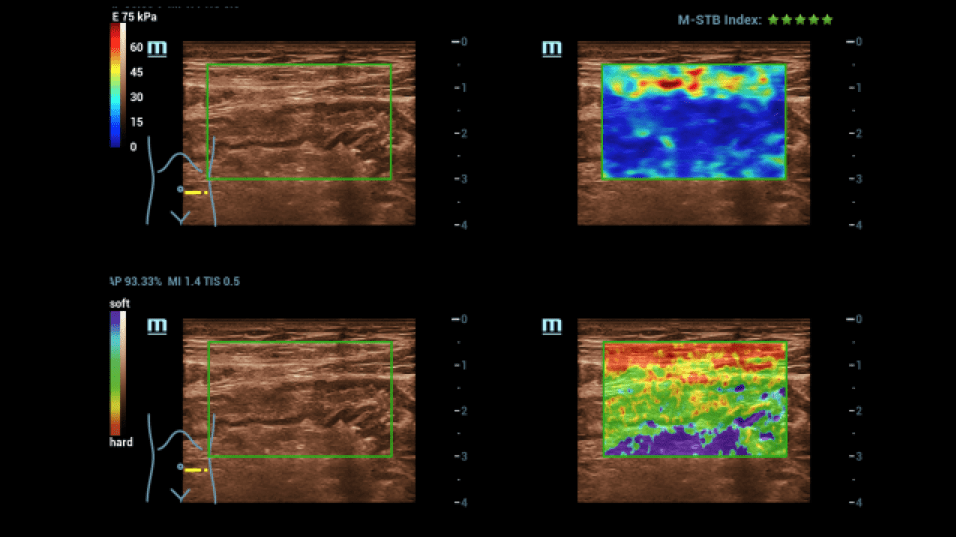
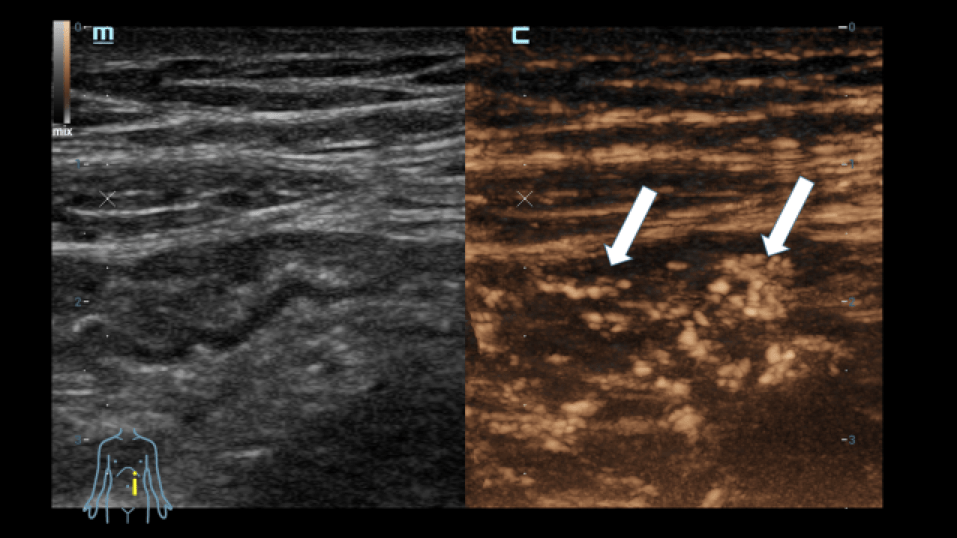
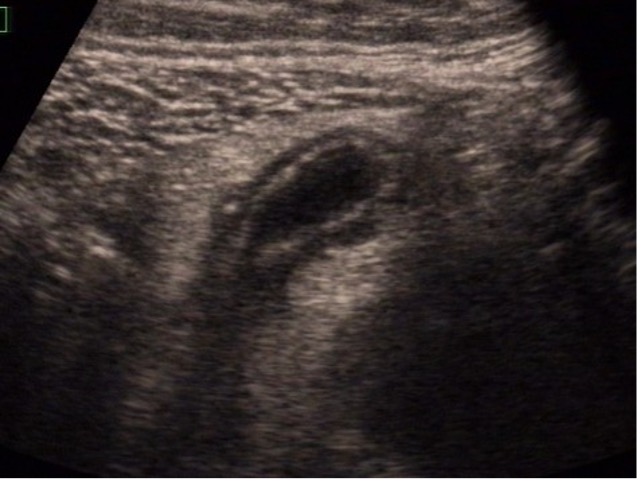
For further clarification, an examination using elastography was carried out (Figure 3):
There were no hardened areas in the area of the conspicuous bowel wall changes. Thus, there was no indication of scars or strictures. A multimodal examination also made it possible to determine possible changes in viscosity as an indication of inflammation. Edematous changes were also visible in sections with interruption of the intestinal wall sections.
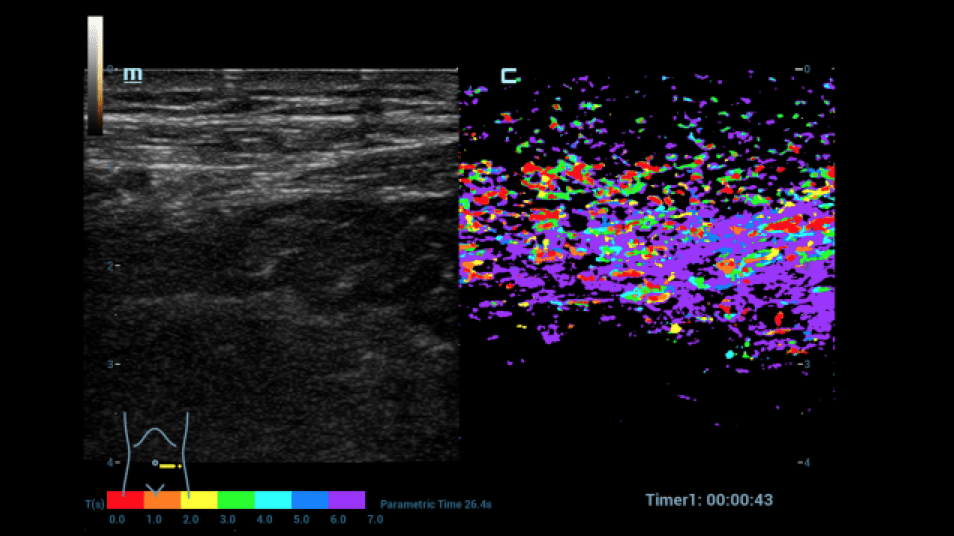
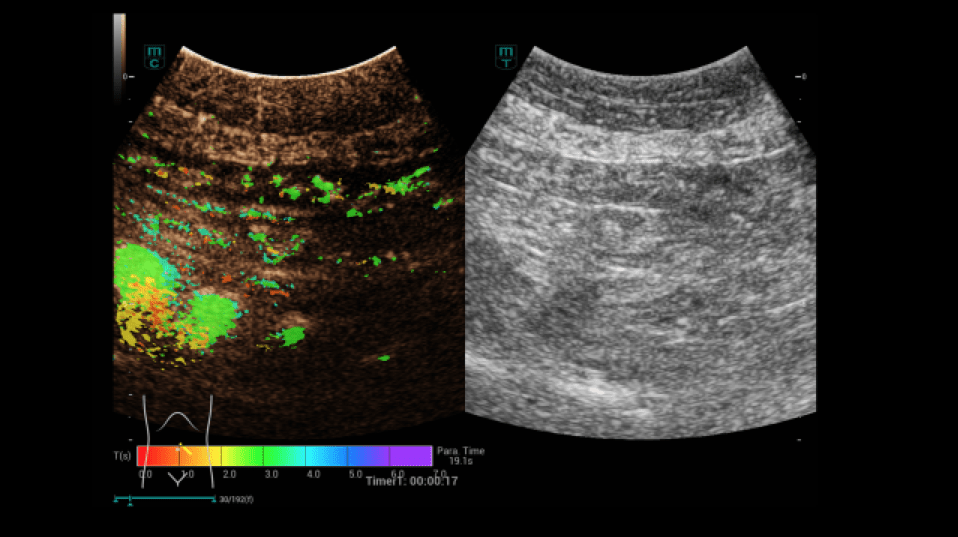
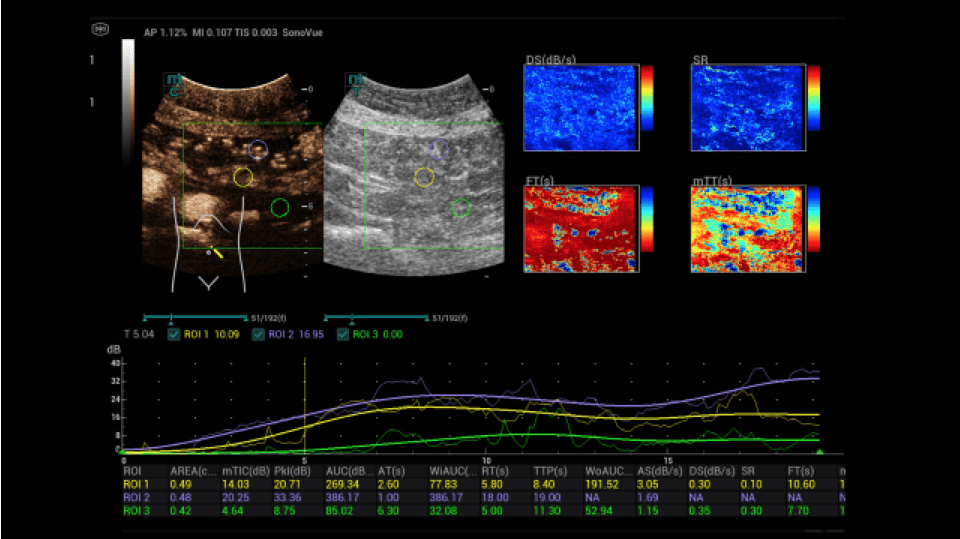
A dynamic contrast-enhanced ultrasound (CEUS) was then performed (Figures 4-9):
After written consent, a bolus of 2.4 ml sulphur hexafluoride microbubbles (SonoVue®) was administered i.v. with 10 ml saline solution via a cubital access. At a low mechanical index (MI < 0.16), the intestine was dynamically examined from the early arterial phase (from 10 to 15 s) to the late phase (after 5 min) with regard to hyperemia and permeability disorders. In addition, the HiFR-CEUS technique was used, with a high level of detail regarding the capillary circulation of the strictly vascular contrast medium. Storage of short cine loops allowed post-processing with perfusion tools with false color analysis.
In the area of the preterminal ileum, there was a rapid transmural accumulation of contrast medium with detectable penetration of microbubbles into the lumen. This was interpreted as a sign of active graft-versus-host disease. After increase immunosuppressive treatment, a follow-up with CEUS and perfusion evaluation was performed. A decrease in hyperemia and transmural penetration could already be demonstrated.
No free fluid was found in the FAST sonography. The abdominal sonography was performed with a convex multifrequency probe (C1-6 MHz) and the detailed assessment of the intestinal tract was additionally performed with a linear multifrequency probe (L6-18 MHz). This revealed an echo increase of the liver tissue in the sense of steatosis without cholestasis with unremarkable blood flow in the CCDS, the size of the spleen was 12x5 cm, there was no urinary retention with slight echo enlargement of a slightly narrowed renal parenchyma, no pathologically enlarged lymph nodes were detectable, the pancreas and gallbladder were not inflammatory. The stomach wall with 6 mm was slightly thickened in the area of the pylorus. The large intestine was clearly distended, which made the examination more difficult. The most striking finding was the ileum part of the small intestine.
ULTRASOUND FINDINGS OF THE SMALL INTESTINE (Figures 1.2):
Particularly in the left middle abdomen, hyperemia with hypercirculation was found in the presence of intestinal wall edema (up to 8 mm) using CCDS. These reactive changes were detectable up to the preterminal ileum. Only slight hypercirculation was detectable mesenteric area. There was no extraluminal fluid content and no retention. The examination revealed no evidence of appendicitis.
This resulted in the following differential diagnoses:
- Bacterial enteritis
- Autoimmune reaction
- Drug-induced colitis
- Viral enteritis
- GvHD (Graft-versus-Host Reaction)
For further clarification, an examination using elastography was carried out (Figure 3):
There were no hardened areas in the area of the conspicuous bowel wall changes. Thus, there was no indication of scars or strictures. A multimodal examination also made it possible to determine possible changes in viscosity as an indication of inflammation. Edematous changes were also visible in sections with interruption of the intestinal wall sections.
A dynamic contrast-enhanced ultrasound (CEUS) was then performed (Figures 4-9):
After written consent, a bolus of 2.4 ml sulphur hexafluoride microbubbles (SonoVue®) was administered i.v. with 10 ml saline solution via a cubital access. At a low mechanical index (MI < 0.16), the intestine was dynamically examined from the early arterial phase (from 10 to 15 s) to the late phase (after 5 min) with regard to hyperemia and permeability disorders. In addition, the HiFR-CEUS technique was used, with a high level of detail regarding the capillary circulation of the strictly vascular contrast medium. Storage of short cine loops allowed post-processing with perfusion tools with false color analysis.
In the area of the preterminal ileum, there was a rapid transmural accumulation of contrast medium with detectable penetration of microbubbles into the lumen. This was interpreted as a sign of active graft-versus-host disease. After increase immunosuppressive treatment, a follow-up with CEUS and perfusion evaluation was performed. A decrease in hyperemia and transmural penetration could already be demonstrated.
3Diagnosis
Active acute intestinal GvHD.
4Discussion
BACKGROUND:
GvHD is still a serious and not uncommon complication of allogeneic stem cell transplantation. The acute form mainly affects skin, liver and the intestine requiring intensified immunosuppression.
CLINICAL PERSPECTIVE:
Continuous monitoring of allogeneic stem cell transplanted patients with regard to the occurrence of intestinal GvHD is imperative, as this can occur at any time through the first year after transplantation.
In addition to laboratory parameters, clinical symptoms and endoscopy, diagnostic imaging procedures (CT, MRI, PET-CT) and CEUS ultrasound diagnostics form the basis for identifying intestinal GvHD. Depending on the method, there are advantages and disadvantages. Perfusion CT would be a fast and accurate method, but comes along with radiation exposure. MRI enterography usually only shows nonspecific hyperemia and is associated with high efforts. The strengths of ultrasound diagnostics lie in the lack of radiation exposure, the comparatively low effort and a method that is quickly available and feasible bedside. However, ultrasound relies on examiner's experience and is in consequence usually available at specialized ultrasound centers only. The potential of CEUS ultrasound diagnostics for detecting GvHD of the intestine has already been repetitively published. Typical findings are a transmural hypoechoic change in the bowel wall with edema >6 mm, band-like soft changes in elastography and hypercirculation in the CEUS with penetration of microbubbles into the lumen. The penetration of microbubbles of an otherwise strictly vascular contrast agent is due to capillary leakage with passage of the microbubbles from the bowel wall into the lumen. With perfusion tools, the decrease in hypercirculation and penetration can also be demonstrated with clinical improvement. High-resolution ultrasound diagnostics can be particularly useful in diagnosing changes up to the preterminal ileum.
THERAPY PLANNING:
The primary goal is to diagnose intestinal aGvHD at onset of symptoms to prevent progression to severe forms by timely start of increased immunosuppression including corticosteroids.
OUTCOME & PROGNOSIS:
Based on the diagnosis of acute intestinal GvHD, the patient received systemic (Prednisolone) and topical (Budesonide) corticosteroid treatment. This resulted in a rapid resolution of the diarrhea and improvement in his general condition. A follow-up CEUS examination 13 days later showed a significant improvement in the intestinal barrier function permitting dose reduction of corticosteroids.
GvHD is still a serious and not uncommon complication of allogeneic stem cell transplantation. The acute form mainly affects skin, liver and the intestine requiring intensified immunosuppression.
CLINICAL PERSPECTIVE:
Continuous monitoring of allogeneic stem cell transplanted patients with regard to the occurrence of intestinal GvHD is imperative, as this can occur at any time through the first year after transplantation.
In addition to laboratory parameters, clinical symptoms and endoscopy, diagnostic imaging procedures (CT, MRI, PET-CT) and CEUS ultrasound diagnostics form the basis for identifying intestinal GvHD. Depending on the method, there are advantages and disadvantages. Perfusion CT would be a fast and accurate method, but comes along with radiation exposure. MRI enterography usually only shows nonspecific hyperemia and is associated with high efforts. The strengths of ultrasound diagnostics lie in the lack of radiation exposure, the comparatively low effort and a method that is quickly available and feasible bedside. However, ultrasound relies on examiner's experience and is in consequence usually available at specialized ultrasound centers only. The potential of CEUS ultrasound diagnostics for detecting GvHD of the intestine has already been repetitively published. Typical findings are a transmural hypoechoic change in the bowel wall with edema >6 mm, band-like soft changes in elastography and hypercirculation in the CEUS with penetration of microbubbles into the lumen. The penetration of microbubbles of an otherwise strictly vascular contrast agent is due to capillary leakage with passage of the microbubbles from the bowel wall into the lumen. With perfusion tools, the decrease in hypercirculation and penetration can also be demonstrated with clinical improvement. High-resolution ultrasound diagnostics can be particularly useful in diagnosing changes up to the preterminal ileum.
THERAPY PLANNING:
The primary goal is to diagnose intestinal aGvHD at onset of symptoms to prevent progression to severe forms by timely start of increased immunosuppression including corticosteroids.
OUTCOME & PROGNOSIS:
Based on the diagnosis of acute intestinal GvHD, the patient received systemic (Prednisolone) and topical (Budesonide) corticosteroid treatment. This resulted in a rapid resolution of the diarrhea and improvement in his general condition. A follow-up CEUS examination 13 days later showed a significant improvement in the intestinal barrier function permitting dose reduction of corticosteroids.
5Teaching Points
With sufficient experience, CEUS ultrasound diagnostics is an adequate diagnostic tool for the diagnosis and potential response assessment of acute intestinal GvHD.
6References
1. Schreyer AG, Landfried K, Jung EM, et al. Contrast-enhanced ultrasound for differential diagnosis of suspected GvHD in patients after allogeneic transplantation. Clin Hemorheol Microcirc. 2011; 49(1-4): 129-36.
2. Pausch AM, Kammerer S, Weber F, et al. Parametric imaging of contrast-enhanced ultrasound (CEUS) for the evaluation of acute gastrointestinal graft-versus-host disease. Cells. 2021; 10(5): 1092.
3. Schreyer AG, Landfried K, Zorger N, et al.. Transmural penetration of intravenously applied microbubbles during contrast-enhanced ultrasound as a new diagnostic feature in patients with GVHD of the bowel. Bone Marrow Transplant. 2011; 46(7): 1006-11.
4. Weber D, Weber M, Hippe K, et al. Non-invasive diagnosis of acute intestinal graft-versus-host disease by a new scoring system using ultrasound morphology, compound elastography, and contrast-enhanced ultrasound. Bone Marrow Transplant. 2019; 54(7): 1038-1048.
5. DGHO. Robert Zeiser, Daniel Wolff, Christoph Scheid, Thomas Luft, Hildegard Greinix, Peter Dreger, Jürgen Finke, Ernst Holler, Jörg Halterfür die DAG-HSZT, Deutsche Arbeitsgemeinschaft für Hämatopoetische Stammzelltransplantation und Zelluläre Therapie e. V. Onkopedia, Graft-versus-Host Erkrankung akut. Available at: https://www.onkopedia.com/de/onkopedia/guidelines/graft-versus-host-erkrankung-akut/@@guideline/html/index.html.
2. Pausch AM, Kammerer S, Weber F, et al. Parametric imaging of contrast-enhanced ultrasound (CEUS) for the evaluation of acute gastrointestinal graft-versus-host disease. Cells. 2021; 10(5): 1092.
3. Schreyer AG, Landfried K, Zorger N, et al.. Transmural penetration of intravenously applied microbubbles during contrast-enhanced ultrasound as a new diagnostic feature in patients with GVHD of the bowel. Bone Marrow Transplant. 2011; 46(7): 1006-11.
4. Weber D, Weber M, Hippe K, et al. Non-invasive diagnosis of acute intestinal graft-versus-host disease by a new scoring system using ultrasound morphology, compound elastography, and contrast-enhanced ultrasound. Bone Marrow Transplant. 2019; 54(7): 1038-1048.
5. DGHO. Robert Zeiser, Daniel Wolff, Christoph Scheid, Thomas Luft, Hildegard Greinix, Peter Dreger, Jürgen Finke, Ernst Holler, Jörg Halterfür die DAG-HSZT, Deutsche Arbeitsgemeinschaft für Hämatopoetische Stammzelltransplantation und Zelluläre Therapie e. V. Onkopedia, Graft-versus-Host Erkrankung akut. Available at: https://www.onkopedia.com/de/onkopedia/guidelines/graft-versus-host-erkrankung-akut/@@guideline/html/index.html.