Student Image Challenge 02
May 21, 2019
Student Image Challenge 03
May 28, 2019A novel use of Intracavity and intravenous CEUS: The delineation of hepatic abscess formation and conformation of catheter placement
AUTHORS:
Mr Stephen Moore:
Sonographer; Princes Royal University Hospital: King’s College NHS Foundation Trust, UK
Professor Paul Sidhu;
Professor of Radiology King’s College Hospital NHS Foundation Trust, UK
Dr Gibran Yusuf
Corresponding Author: Stephen Moore s.moore11@nhs.net
Mr Stephen Moore:
Sonographer; Princes Royal University Hospital: King’s College NHS Foundation Trust, UK
Professor Paul Sidhu;
Professor of Radiology King’s College Hospital NHS Foundation Trust, UK
Dr Gibran Yusuf
Corresponding Author: Stephen Moore s.moore11@nhs.net
1Case Report
A 56 year old male presented in the emergency department with fever, rigors and hypotension. Initial biochemistry demonstrated deranged liver function tests and gram-negative bacilli for which he was been managed with antibiotics. The patient had a medical history of liver transplantation complicated by hepatic artery thrombosis following a hepatic artery conduit formation. A hepatic abscess was a possibility, with Initial concerns for an ischemic cause of abscess formation.
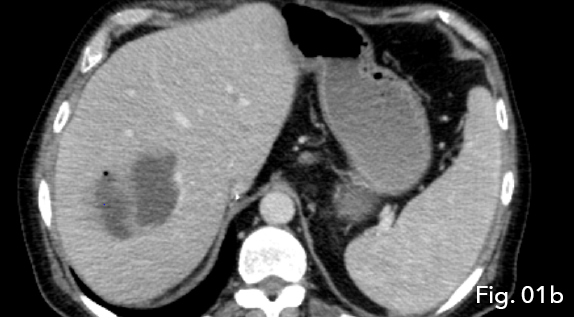
CT demonstrated intrahepatic blood flow via arterial collateralisation secondary to occlusion of the hepatic artery at the level of anastomosis proper as well as occlusion of the conduit vessel. A multiloculated, peripherally enhancing lesion with a low density centre and single gaseous loculation was seen in segments V and VIII of the liver. Due to the acute onset of clinical symptoms and new imaging findings, abscess formation secondary to ischemic liver disease was concluded (Fig 1a and 1b).
Ultrasound guided drainage was undertaken which demonstrated gas formation within the abscess with both solid and cystic components on B-mode imaging. Only 10 ml of pus was aspirated and IV antibiotics were instituted to control sepsis.
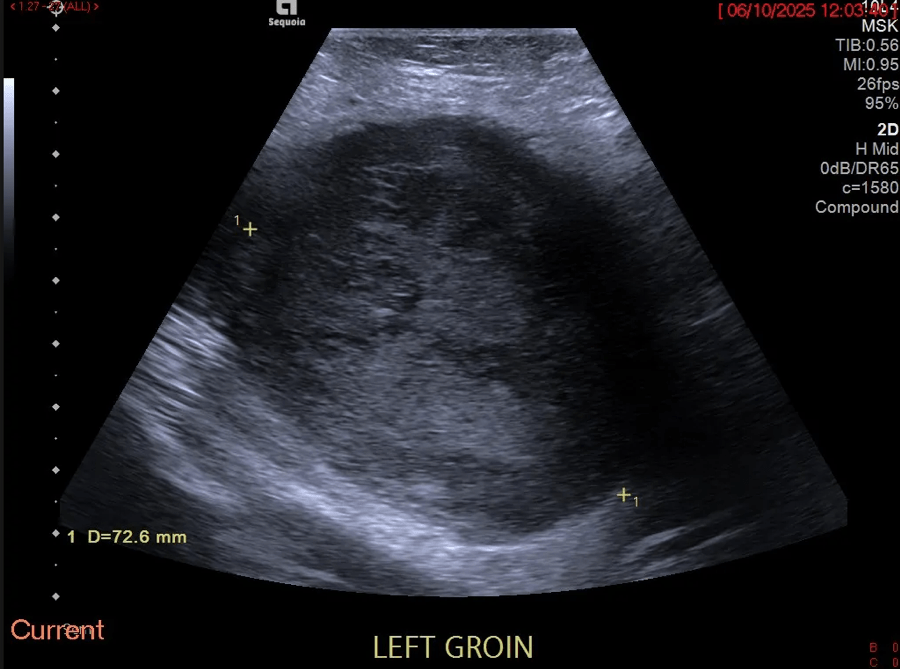
When there was minimal drainage from the catheter, a repeat ultrasound was undertaken to assess resolution. B-mode ultrasound demonstrated a solid low reflective area, presumed to be an abscess with a drainage catheter correctly positioned (Fig 2). The abscess had reduced in size in comparison with the previous examination, however, a new hypoechoic region was noted superiorly to the first abscess (Fig 3) giving rise to suspicion of new abscess formation. IV and IC CEUS were undertaken following local protocols. IV CEUS utilised 2.4 ml of SonoVue (Bracco, Milan) administered through a cannula situated in the left antecubital fossa, followed by a 10ml bolus of saline solution. IC CEUS utilised 0.1 ml of SonoVue (Bracco, Milan) diluted in 50 ml of saline administered via the hepatic drainage catheter.
IV CEUS interrogation of the new second hypoechoic region superior to the primary abscess demonstrated features consistent with a hepatic abscess formation. IC CEUS demonstrated intracavitary communication between the two regions of abscess formation helping confirm the findings as a single abscess (Fig 4), negating the need for a second percutaneous hepatic drainage catheter. Furthermore the combination of IV and IC CEUS concluded that true fluid components were still present and the true size of the abscess cavity was much smaller than B-mode ultrasound had suggested (Fig 5).
CT demonstrated intrahepatic blood flow via arterial collateralisation secondary to occlusion of the hepatic artery at the level of anastomosis proper as well as occlusion of the conduit vessel. A multiloculated, peripherally enhancing lesion with a low density centre and single gaseous loculation was seen in segments V and VIII of the liver. Due to the acute onset of clinical symptoms and new imaging findings, abscess formation secondary to ischemic liver disease was concluded (Fig 1a and 1b).
Ultrasound guided drainage was undertaken which demonstrated gas formation within the abscess with both solid and cystic components on B-mode imaging. Only 10 ml of pus was aspirated and IV antibiotics were instituted to control sepsis.
When there was minimal drainage from the catheter, a repeat ultrasound was undertaken to assess resolution. B-mode ultrasound demonstrated a solid low reflective area, presumed to be an abscess with a drainage catheter correctly positioned (Fig 2). The abscess had reduced in size in comparison with the previous examination, however, a new hypoechoic region was noted superiorly to the first abscess (Fig 3) giving rise to suspicion of new abscess formation. IV and IC CEUS were undertaken following local protocols. IV CEUS utilised 2.4 ml of SonoVue (Bracco, Milan) administered through a cannula situated in the left antecubital fossa, followed by a 10ml bolus of saline solution. IC CEUS utilised 0.1 ml of SonoVue (Bracco, Milan) diluted in 50 ml of saline administered via the hepatic drainage catheter.
IV CEUS interrogation of the new second hypoechoic region superior to the primary abscess demonstrated features consistent with a hepatic abscess formation. IC CEUS demonstrated intracavitary communication between the two regions of abscess formation helping confirm the findings as a single abscess (Fig 4), negating the need for a second percutaneous hepatic drainage catheter. Furthermore the combination of IV and IC CEUS concluded that true fluid components were still present and the true size of the abscess cavity was much smaller than B-mode ultrasound had suggested (Fig 5).
2Discussion
Liver transplant carries a 4-15% risk of hepatic artery thrombosis (5). While these complications are closely associated to rejection of the transplanted liver, collateralisation can rarely allow adequate perfusion of the liver negating the need for re-transplantation. However, there is increased risk of ischemic liver disease (6) and hepatic abscess formation, arising from breakdown of the bile ducts, which are solely supplied by the hepatic artery following transplantation (5,6).
Hepatic abscess historically had a mortality rate of 75-80% which in recent years has been reduced to 10-40% (7). It is imperative that hepatic abscesses are appropriately managed with antibiotics and where necessary percutaneous drainage (8).
IV CEUS has been well documented for the detection of hepatic abscesses (1,2) and the use of CEUS to identify, characterise and monitor hepatic abscesses is supported by both EFSUMB and WFUMB (3). CEUS possesses several advantages over Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) by being radiation free, cost-effective and portable (6). CEUS has an excellent safety profile being non-nephrotoxic allowing it to be used in patients with renal impairment (6). The adverse reaction rates of CEUS are comparable to commonplace antibiotics and less than those used in CT or MRI (9).
CEUS features of a hepatic abscess are peripheral enhancement with no uptake visible in the centrally necrotic or fluid components, more complex cases may also demonstrate internal septation enhancement and stranding (3,10,11). IV CEUS is advantageous over B-mode ultrasound as the contrast helps increase lesion delineation and demonstrates true fluid components of the abscess which may appear solid on B-mode imaging. This is due to low-level echogenic debris within the abscess cavity which may mimic solid tissues within the abscess (3). The demonstration of true fluid components and real-time imaging allows CEUS guidance of interventional drainage placement (12). CEUS guided drainage allows increased accuracy by allowing the clinician to target true fluid areas directly (12). Once a drainage catheter is in-situ CEUS can be used to monitor resolution.
Recent literature shows an increase in the use of IC CEUS (4). The purely intravascular nature of CEUS ensures that once CEUS is administered into a free space, the contrast will be retained within the localised cavity (4). Utilising this; IC CEUS is able to demonstrate the true extent of a cavity, any intercavitary communications and confirm correct drainage catheter placement (4).
This case demonstrates the use of ultrasound to monitor abscess resolution with the discovery of what B-mode ultrasound previously documented as a solid primary abscess with a potential new, secondary abscess. The use of IV CEUS demonstrated features conclusive of a new, small abscess; IV contrast also demonstrated that both the new and old abscess still had fluid components amenable to continued drainage.
While IV CEUS had confirmed two areas of abscess formation and that the true fluid components persisted which were still amenable to drainage; IC CEUS was undertaken following local protocols to confirm that the original percutaneous hepatic drainage catheter was; still in-situ, functioning and to see if there was any intracavitary communications between the primary and the new abscess.
IC CEUS confirmed that the hepatic drainage catheter was correctly sited and that the two abscess regions were connected via intracavitary channels/ communications, assuring the clinicians that the original drainage catheter was sufficient and functioning, negating the need for repositioning the original drainage catheter and negating the need for a second percutaneous hepatic drainage catheter.
Hepatic abscess historically had a mortality rate of 75-80% which in recent years has been reduced to 10-40% (7). It is imperative that hepatic abscesses are appropriately managed with antibiotics and where necessary percutaneous drainage (8).
IV CEUS has been well documented for the detection of hepatic abscesses (1,2) and the use of CEUS to identify, characterise and monitor hepatic abscesses is supported by both EFSUMB and WFUMB (3). CEUS possesses several advantages over Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) by being radiation free, cost-effective and portable (6). CEUS has an excellent safety profile being non-nephrotoxic allowing it to be used in patients with renal impairment (6). The adverse reaction rates of CEUS are comparable to commonplace antibiotics and less than those used in CT or MRI (9).
CEUS features of a hepatic abscess are peripheral enhancement with no uptake visible in the centrally necrotic or fluid components, more complex cases may also demonstrate internal septation enhancement and stranding (3,10,11). IV CEUS is advantageous over B-mode ultrasound as the contrast helps increase lesion delineation and demonstrates true fluid components of the abscess which may appear solid on B-mode imaging. This is due to low-level echogenic debris within the abscess cavity which may mimic solid tissues within the abscess (3). The demonstration of true fluid components and real-time imaging allows CEUS guidance of interventional drainage placement (12). CEUS guided drainage allows increased accuracy by allowing the clinician to target true fluid areas directly (12). Once a drainage catheter is in-situ CEUS can be used to monitor resolution.
Recent literature shows an increase in the use of IC CEUS (4). The purely intravascular nature of CEUS ensures that once CEUS is administered into a free space, the contrast will be retained within the localised cavity (4). Utilising this; IC CEUS is able to demonstrate the true extent of a cavity, any intercavitary communications and confirm correct drainage catheter placement (4).
This case demonstrates the use of ultrasound to monitor abscess resolution with the discovery of what B-mode ultrasound previously documented as a solid primary abscess with a potential new, secondary abscess. The use of IV CEUS demonstrated features conclusive of a new, small abscess; IV contrast also demonstrated that both the new and old abscess still had fluid components amenable to continued drainage.
While IV CEUS had confirmed two areas of abscess formation and that the true fluid components persisted which were still amenable to drainage; IC CEUS was undertaken following local protocols to confirm that the original percutaneous hepatic drainage catheter was; still in-situ, functioning and to see if there was any intracavitary communications between the primary and the new abscess.
IC CEUS confirmed that the hepatic drainage catheter was correctly sited and that the two abscess regions were connected via intracavitary channels/ communications, assuring the clinicians that the original drainage catheter was sufficient and functioning, negating the need for repositioning the original drainage catheter and negating the need for a second percutaneous hepatic drainage catheter.
3Conclusion
This case demonstrates concordance with current literature regarding how IV CEUS can confidently identify abscess formation and differentiate true fluid regions from solid areas. Moreover, this case demonstrates how IC CEUS can help confirm communication between abscess cavities and how IC CEUS can confirm correct drainage catheter placement.
The authors hypothesise that the utilisation of both IV and IC CEUS could assist in appropriate management of patients presenting with suspected multiple hepatic abscesses; allowing for less invasive treatments where clinically appropriate.
4Figure Legends
Fig 1a: Abscess formation was seen within the liver, indicating abscess formation secondary to ischaemic liver disease (star).
Figure 1b: Note the multiloculated/ spectated nature of the low density lesion (star) with gaseous loculation seen in the non-gravity dependent portion of the abscess (arrow).
Fi 2: Note the hypoechoic but more solid appearance of the abscess (star) with the catheter tip seen within (arrow).
Fig 3: The abscess had reduced in size in comparison with the previous examination (star), however, new hypoechoic region was noted superiorly to the first abscess (arrow).
Fig 4: IC CEUS demonstrating intracavitary communications (arrows) between the two abscess cavities helping confirm the findings as a single abscess
Fig 5: IV and IC CEUS concluded the true fluid components were still present and the true size of the abscess cavity was much smaller than B-mode ultrasound suggested (star).
Figure 1b: Note the multiloculated/ spectated nature of the low density lesion (star) with gaseous loculation seen in the non-gravity dependent portion of the abscess (arrow).
Fi 2: Note the hypoechoic but more solid appearance of the abscess (star) with the catheter tip seen within (arrow).
Fig 3: The abscess had reduced in size in comparison with the previous examination (star), however, new hypoechoic region was noted superiorly to the first abscess (arrow).
Fig 4: IC CEUS demonstrating intracavitary communications (arrows) between the two abscess cavities helping confirm the findings as a single abscess
Fig 5: IV and IC CEUS concluded the true fluid components were still present and the true size of the abscess cavity was much smaller than B-mode ultrasound suggested (star).
5References
1. Kishina, M., Koda, M., Tokunaga, S., Miyoshi, K., Fujise, Y., Kato, J., Matono, T., Sugihara, T., Murawaki, Y. (2015) ‘Usefulness of contrast-enhanced ultrasound with Sonazoid for evaluating liver abscess in comparison with conventional B-mode ultrasound’, Hepatology Research, 45(3), pp. 337-342 doi: 10.1111/hepr.1234.
2. Huang, DY., Yusuf, GT., Daneshi, M., Ramnarine, R., Deganello, A., Sellars, ME., Sidhu, PS. (2018) ‘Contrast-enhanced ultrasound (CEUS) in abdominal intervention’, Abdominal Radiology (New York), 43(4), pp. 960-976 doi: 10.1007/s00261-01801473-8.
3. Claudon, M., Dietrich, CF., Choi, BI., Cosgrove, DO., Kudo, M., Nolsøe, CP., Piscaglia, F., Wilson, SR., Barr., RG., Chammas, MC., Chaubal, NG., Chen, MH., Clevert, DA., Correas, JM., Ding, H., Forsberg, F., Fowlkes, JB., Gibson, RN., Goldberg, BB., Lassau, N., Mattrey, RF., Moriyasu, F., Solbiati, L., Weskott, HP., Xu, HX. (2012) Ultraschall in der Medizin / European Journal of Ultrasound, 34(1), pp. 11-29 doi:10.1055/s-0032-1325499.
4. Deganello, A., Rafailidis, V., Sellars, ME., Ntoulia, A., Kalogerakou, K., Ruiz, G., Cosgrove, DO., Sidhu, PS. (2017) ‘Intravenous and Intracavitary Use of Contrast-Enhances Ultrasound in the Evaluation and Management of Complicated Pediatric Pneumonia’, Journal of Ultrasound in Medicine, 36(9), pp. 1943-1954 doi: 10.1002/jum.14269.
5. Puliti Reigada, CH., de Ataide, EC., de Almeida Prado Mattosinho, T., Boin, IFSF. (2017) ‘Hepatic Artery Thrombosis After Liver Transplantation: Five-Year Experience at the State University of Campinas’, Transplantation Proceedings, 49(4), pp. 867-870 doi:10.1016/j.transproceed.2017.01.056.
6. Ma, L., Qiang, L., Luo, Y. (2016) ‘Vascular complications after adult living donor liver transplantation: Evaluation with ultrasonography’, World Journal of Gastroenterology, 22(4), pp. 1617-1626 doi: 10.3748/wjg.v22.i4.1617.
7. Mavilia, MG,. Molina, M., Wu, GY. (2016) ‘The Evolving Nature of Hepatic Abscess: A Review’, Journal of Clinical and Translational Hepatology, 4(2), pp. 158-168 doi:10.14218/JCTH.2016.00004.
8. Singh, S., Chaudhary, P., Saxena, N., Khandelwal, S., Poddar, DD., Biswal, UC. (2013) ‘Treatment of liver abscess: prospective randomized comparison of catheter drainage and needle aspiration’, Annals of Gastroenterology, 26(4), pp. 332-339
9. Marshall, G., Sykes, A., Berry, j., Jonker, L. (2011) ‘The “humble” bubble: Contrast-enhances ultrasound’, Radiography, 17(4), pp. 345-349 doi: https://doi.org/10.1016/j.radi.2011.05.002.
10. Feier, D., Socaciu, M., Anton, O., Al Hajjar, N., Badea, R. (2012) ‘The combined role of intravenous contrast enhanced ultrasound (CEUS) and computed tomography (CT) in liver abscess diagnosis’, Chirurgia, 107(3), pp. 343-351
11. Catalano, O., Sandomenico, F., Mattace, RM., Siani, A. (2004) ‘Low Mechanical Index Contrast-Enhanced Sonographic Findings of Pyogenic Hepatic Abscesses’, American Journal of Roentgenology, 182(2), pp. 447-450 doi: 10.2214/ajr.182.2.1820447.
12. Huang, DY., Yusuf, GT., Daneshi, M., Husainy, MA., Ramnarine, R., Sellars, MEK., Sidhu PS. (2017) ‘Contrast-enhanced US-guided Interventions: Improving Success Rate and Avoiding Complications Using US Contrast Agents’, Radiographics, 37(2), pp. 652-664 doi: 10.1148/rg.2017160123.
2. Huang, DY., Yusuf, GT., Daneshi, M., Ramnarine, R., Deganello, A., Sellars, ME., Sidhu, PS. (2018) ‘Contrast-enhanced ultrasound (CEUS) in abdominal intervention’, Abdominal Radiology (New York), 43(4), pp. 960-976 doi: 10.1007/s00261-01801473-8.
3. Claudon, M., Dietrich, CF., Choi, BI., Cosgrove, DO., Kudo, M., Nolsøe, CP., Piscaglia, F., Wilson, SR., Barr., RG., Chammas, MC., Chaubal, NG., Chen, MH., Clevert, DA., Correas, JM., Ding, H., Forsberg, F., Fowlkes, JB., Gibson, RN., Goldberg, BB., Lassau, N., Mattrey, RF., Moriyasu, F., Solbiati, L., Weskott, HP., Xu, HX. (2012) Ultraschall in der Medizin / European Journal of Ultrasound, 34(1), pp. 11-29 doi:10.1055/s-0032-1325499.
4. Deganello, A., Rafailidis, V., Sellars, ME., Ntoulia, A., Kalogerakou, K., Ruiz, G., Cosgrove, DO., Sidhu, PS. (2017) ‘Intravenous and Intracavitary Use of Contrast-Enhances Ultrasound in the Evaluation and Management of Complicated Pediatric Pneumonia’, Journal of Ultrasound in Medicine, 36(9), pp. 1943-1954 doi: 10.1002/jum.14269.
5. Puliti Reigada, CH., de Ataide, EC., de Almeida Prado Mattosinho, T., Boin, IFSF. (2017) ‘Hepatic Artery Thrombosis After Liver Transplantation: Five-Year Experience at the State University of Campinas’, Transplantation Proceedings, 49(4), pp. 867-870 doi:10.1016/j.transproceed.2017.01.056.
6. Ma, L., Qiang, L., Luo, Y. (2016) ‘Vascular complications after adult living donor liver transplantation: Evaluation with ultrasonography’, World Journal of Gastroenterology, 22(4), pp. 1617-1626 doi: 10.3748/wjg.v22.i4.1617.
7. Mavilia, MG,. Molina, M., Wu, GY. (2016) ‘The Evolving Nature of Hepatic Abscess: A Review’, Journal of Clinical and Translational Hepatology, 4(2), pp. 158-168 doi:10.14218/JCTH.2016.00004.
8. Singh, S., Chaudhary, P., Saxena, N., Khandelwal, S., Poddar, DD., Biswal, UC. (2013) ‘Treatment of liver abscess: prospective randomized comparison of catheter drainage and needle aspiration’, Annals of Gastroenterology, 26(4), pp. 332-339
9. Marshall, G., Sykes, A., Berry, j., Jonker, L. (2011) ‘The “humble” bubble: Contrast-enhances ultrasound’, Radiography, 17(4), pp. 345-349 doi: https://doi.org/10.1016/j.radi.2011.05.002.
10. Feier, D., Socaciu, M., Anton, O., Al Hajjar, N., Badea, R. (2012) ‘The combined role of intravenous contrast enhanced ultrasound (CEUS) and computed tomography (CT) in liver abscess diagnosis’, Chirurgia, 107(3), pp. 343-351
11. Catalano, O., Sandomenico, F., Mattace, RM., Siani, A. (2004) ‘Low Mechanical Index Contrast-Enhanced Sonographic Findings of Pyogenic Hepatic Abscesses’, American Journal of Roentgenology, 182(2), pp. 447-450 doi: 10.2214/ajr.182.2.1820447.
12. Huang, DY., Yusuf, GT., Daneshi, M., Husainy, MA., Ramnarine, R., Sellars, MEK., Sidhu PS. (2017) ‘Contrast-enhanced US-guided Interventions: Improving Success Rate and Avoiding Complications Using US Contrast Agents’, Radiographics, 37(2), pp. 652-664 doi: 10.1148/rg.2017160123.


![A novel use of Intracavity and intravenous CEUS: The delineation of hepatic abscess formation and conformation of catheter placement. </br> [May 2019]](https://efsumb.org/wp-content/uploads/2020/11/cotm-may2019-1a.png)
![A novel use of Intracavity and intravenous CEUS: The delineation of hepatic abscess formation and conformation of catheter placement. </br> [May 2019]](https://efsumb.org/wp-content/uploads/2020/11/cotm-may2019-2.png)
![A novel use of Intracavity and intravenous CEUS: The delineation of hepatic abscess formation and conformation of catheter placement. </br> [May 2019]](https://efsumb.org/wp-content/uploads/2020/11/cotm-may2019-3.png)
![A novel use of Intracavity and intravenous CEUS: The delineation of hepatic abscess formation and conformation of catheter placement. </br> [May 2019]](https://efsumb.org/wp-content/uploads/2020/11/cotm-may2019-4.png)
![A novel use of Intracavity and intravenous CEUS: The delineation of hepatic abscess formation and conformation of catheter placement. </br> [May 2019]](https://efsumb.org/wp-content/uploads/2020/11/cotm-may2019-5.png)