- European Federation of Societies for Ultrasound in Medicine and Biology ~ Educating all for competence to practice ultrasound safely
Myotendinous rupture of the distal biceps [Jun 2018]
June 13, 2018
Gallbladder Perforation and hepatic abscess formation in Advanced Cholecystitis: CEUS as an Adjunct [Aug 2018]
August 16, 2018A rare cause for delayed renal transplant graft function
Aia Mehdi, Philippa Lee, Ali Alsafi, Chris Harvey,
Imperial College Healthcare Trust, London
Imperial College Healthcare Trust, London
1Clinical History
A 52-year-old male patient with a four year history of end-stage renal failure secondary to Systemic Lupus Erythematosus (SLE) with renal involvement had received a cadaveric donor renal transplant. Of note, he had a past medical history of heart transplantation two decades prior as a result of cardiac involvement secondary to SLE as well as multiple pulmonary emboli and was on lifelong anticoagulation.
In the acute post-operative period, his serum creatinine remained high, he was oliguric and hypervolaemic demonstrating delayed renal graft function. The most likely differentials were considered - obstruction, compressive collection, arterial or venous thrombosis and pyelonephritis- and excluded by unremarkable serial ultrasounds and normal inflammatory markers.
In the acute post-operative period, his serum creatinine remained high, he was oliguric and hypervolaemic demonstrating delayed renal graft function. The most likely differentials were considered - obstruction, compressive collection, arterial or venous thrombosis and pyelonephritis- and excluded by unremarkable serial ultrasounds and normal inflammatory markers.
2Imaging Findings
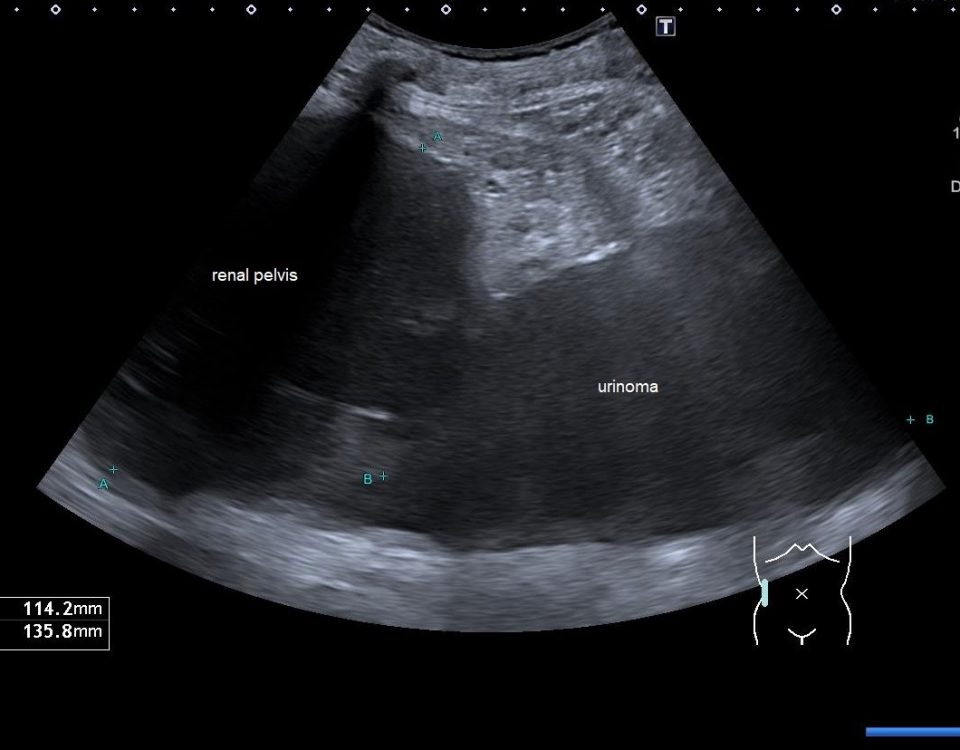
Post-operative serial ultrasounds demonstrated unremarkable cortico-medullary differentiation and parenchymal echogenicity, Colour and power Doppler demonstrated an apparently well-perfused, non-obstructed graft (Fig 1 & 2).
Subsequently, contrast-enhanced US (CEUS) of the renal transplant graft revealed a subcortical rim of hypoperfusion in keeping with cortical necrosis (Fig 3). In retrospect there is a very subtle rim of echopoor change seen in this site on the Power and Colour Doppler but CEUS makes it much more conspicuous.
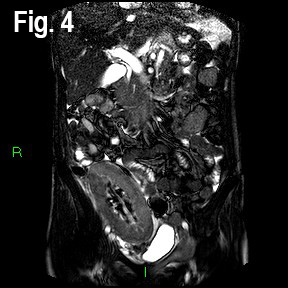
A Magnetic Resonance Angiogram (MRA) showed a low cortical signal and non-enhancing cortex post intravenous Gadinolinium administration, but no renal artery or vein thrombosis or stenosis (Fig 4 & 5).
Subsequently, contrast-enhanced US (CEUS) of the renal transplant graft revealed a subcortical rim of hypoperfusion in keeping with cortical necrosis (Fig 3). In retrospect there is a very subtle rim of echopoor change seen in this site on the Power and Colour Doppler but CEUS makes it much more conspicuous.
A Magnetic Resonance Angiogram (MRA) showed a low cortical signal and non-enhancing cortex post intravenous Gadinolinium administration, but no renal artery or vein thrombosis or stenosis (Fig 4 & 5).
3Diagnosis
Renal cortical necrosis of transplant kidney
4Discussion
Renal cortical necrosis (RCN) is a rare cause of acute renal failure and is due to significantly prolonged diminished renal arterial perfusion secondary to vasospasm, microvascular injury or intravascular thrombosis. The process usually affects the small intra-cortical vessels, interlobular and afferent arterioles. Typically the arcuate arteries are spared, although they may also be affected (1).
The pathogenesis of RCN is not fully understood, although the final common pathway is microvascular hypoperfusion secondary to endothelial injury. This can occur in pregnancy (for example in placental abruption, placenta praevia or eclampsia), haemodynamic shock (due to sepsis, trauma, hypovolaemia) disseminated intravascular coagulation, acute transplant rejection and haemolytic uraemic syndrome (2-9) and various other pathologies which may contribute to these states.
Clinical presentation is usually with acute renal failure in native kidneys or transplant dysfunction as demonstrated by flank pain, haematuria, rising creatinine, oliguria and hypervolaemia. If left untreated or treatment is delayed, acute renal failure secondary to RCN has a high mortality rate.
RCN is difficult to appreciate on conventional and Doppler ultrasound as these modes can only detect signals from vessels down to approximately 1mm in diameter. CEUS has a higher sensitivity and enables assessment of the renal microcirculation, detecting signals from vessels of the order of 100µm in diameter. (10) Typically, on B mode the whole kidney may be swollen with reduced or absent flow peripherally. Resistive indices may be elevated. Contrast-enhanced CT and MR may show a thin rim of enhancing subcapsular tissue (‘rim sign’) due to subcapsular collateral supply. Low signal of the inner renal cortex and columns of Bertin may be seen on T1 and T2 sequences. Dystrophic calcification will eventually occur but is a late sign (11). However, both iodinated and gadolinium contrast agents may be potentially deleterious in renal failure and should ideally be avoided. Biopsy is the gold standard for diagnosis of RCN. In our patient, a biopsy of the transplant kidney demonstrated cortical infarction secondary to thrombotic microangiopathy and small vessel thrombosis. The cause of the thrombotic microangiopathy was believed to be due to a combination of predisposing factors including SLE, previously demonstrated thromboembolic tendancy in addition to possible acute rejection. He received treatment with plasma exchange and intravenous immunoglobulin therapy with subsequent improvement in renal function.
The pathogenesis of RCN is not fully understood, although the final common pathway is microvascular hypoperfusion secondary to endothelial injury. This can occur in pregnancy (for example in placental abruption, placenta praevia or eclampsia), haemodynamic shock (due to sepsis, trauma, hypovolaemia) disseminated intravascular coagulation, acute transplant rejection and haemolytic uraemic syndrome (2-9) and various other pathologies which may contribute to these states.
Clinical presentation is usually with acute renal failure in native kidneys or transplant dysfunction as demonstrated by flank pain, haematuria, rising creatinine, oliguria and hypervolaemia. If left untreated or treatment is delayed, acute renal failure secondary to RCN has a high mortality rate.
RCN is difficult to appreciate on conventional and Doppler ultrasound as these modes can only detect signals from vessels down to approximately 1mm in diameter. CEUS has a higher sensitivity and enables assessment of the renal microcirculation, detecting signals from vessels of the order of 100µm in diameter. (10) Typically, on B mode the whole kidney may be swollen with reduced or absent flow peripherally. Resistive indices may be elevated. Contrast-enhanced CT and MR may show a thin rim of enhancing subcapsular tissue (‘rim sign’) due to subcapsular collateral supply. Low signal of the inner renal cortex and columns of Bertin may be seen on T1 and T2 sequences. Dystrophic calcification will eventually occur but is a late sign (11). However, both iodinated and gadolinium contrast agents may be potentially deleterious in renal failure and should ideally be avoided. Biopsy is the gold standard for diagnosis of RCN. In our patient, a biopsy of the transplant kidney demonstrated cortical infarction secondary to thrombotic microangiopathy and small vessel thrombosis. The cause of the thrombotic microangiopathy was believed to be due to a combination of predisposing factors including SLE, previously demonstrated thromboembolic tendancy in addition to possible acute rejection. He received treatment with plasma exchange and intravenous immunoglobulin therapy with subsequent improvement in renal function.
5Teaching Points
RCN is a rare but important cause of acute renal failure and transplant dysfunction and can pose a diagnostic challenge on B mode and Doppler ultrasound, which may be normal.
Microbubble CEUS should be considered in patients with suspected RCN for early diagnosis and for follow-up. It is a safe, non-nephrotoxic, inexpensive and easy to use diagnostic tool, which obviates the need for the use of intravenous iodinated contrast, which may be detrimental in acute renal failure (12, 13).
Microbubble CEUS should be considered in patients with suspected RCN for early diagnosis and for follow-up. It is a safe, non-nephrotoxic, inexpensive and easy to use diagnostic tool, which obviates the need for the use of intravenous iodinated contrast, which may be detrimental in acute renal failure (12, 13).
6References
(1) Tovbin, D., Lantsberg, S., Feldman, L., et al., Unilateral acute renal cortical necrosis (ACN) following skipping with a rope. Nephrology Dialysis Transplantation, 2000. 15 (3): 415-418.
(2) Chugh, K.S., Jha, V., Sakhuja V., et al., Acute renal cortical necrosis—a study of 113 patients. Ren Fail 1994. 16 (1): 37–47.
(3) Blumhardt, R., Growcock, G. and Lasher, J.C., Cortical necrosis in a renal transplant. AJR Am J Roentgenol , 1982. 141 (1): 95–96.
(4) Prakash, J., Vohra, R., Wani, I.A., et al., Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: a single-centre experience of 22 years from Eastern India. Nephrol Dial Transplant, 2007. 4: 1213–1217.
(5) Frimat, M., Decambron, M., Lebas C., et al., Renal Cortical Necrosis in Postpartum Hemorrhage: A Case Series. Am J Kidney Dis, 2016. 68 (1): 50-57.
(6) Huang, C.C., and Huang J.K., Sepsis-induced acute bilateral renal cortical necrosis. Nephrology (Carlton), 2011. 16(8): 787.
(7) Lee, J.W., Won, N.H., Cho, e., et al., Postoperative hemolytic uremic syndrome with renal cortical necrosis following laparoscopic hemicolectomy. Ren Fail, 2013. 35(5): 725-8.
(8) Shiradhonkar, S., Jha, R., Rao, B.S., et al., Acute cortical necrosis following renal transplantation in a case of sickle cell trait. Indian J Nephrol, 2011. 21(4): 286-8.
(9) Quintana, L.F., Cofan F., Reverte J.C., et al., Renal cortical necrosis after kidney transplantation associated with the prothrombin 20210A mutation. Nephrol Dial Transplant, 2006. 21(5): 1455-6.
(10) Sidhu, P.S., Cantisani, V., Dietrich, C.F., et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS) in non-hepatic applications. Update 2017. Ultraschall Med, 2018. 39: 2–44.
(11) Kim, H.J., and Cho, O.K., CT scan as an important diagnostic tool in the initial phase of diffuse bilateral renal cortical necrosis. Clin Nephrol, 1996. 45 (2): 125-30.
(12) Fernandez, C.P., Ripolles, T., Martinez M.J., et al., Diagnosis of acute cortical necrosis in renal transplantation by contrast-enhanced ultrasound: a preliminary experience. Ultraschall Med, 2013. 34(4): 340-4.
(13) Harvey, C.J., Alsafi, A., Kuzmich, S., et al., Role of US Contrast Agents in the Assessment of Indeterminate Solid and Cystic Lesions in Native and Transplant Kidneys. Radiographics, 2015. 35(5): 1419-30.
(2) Chugh, K.S., Jha, V., Sakhuja V., et al., Acute renal cortical necrosis—a study of 113 patients. Ren Fail 1994. 16 (1): 37–47.
(3) Blumhardt, R., Growcock, G. and Lasher, J.C., Cortical necrosis in a renal transplant. AJR Am J Roentgenol , 1982. 141 (1): 95–96.
(4) Prakash, J., Vohra, R., Wani, I.A., et al., Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: a single-centre experience of 22 years from Eastern India. Nephrol Dial Transplant, 2007. 4: 1213–1217.
(5) Frimat, M., Decambron, M., Lebas C., et al., Renal Cortical Necrosis in Postpartum Hemorrhage: A Case Series. Am J Kidney Dis, 2016. 68 (1): 50-57.
(6) Huang, C.C., and Huang J.K., Sepsis-induced acute bilateral renal cortical necrosis. Nephrology (Carlton), 2011. 16(8): 787.
(7) Lee, J.W., Won, N.H., Cho, e., et al., Postoperative hemolytic uremic syndrome with renal cortical necrosis following laparoscopic hemicolectomy. Ren Fail, 2013. 35(5): 725-8.
(8) Shiradhonkar, S., Jha, R., Rao, B.S., et al., Acute cortical necrosis following renal transplantation in a case of sickle cell trait. Indian J Nephrol, 2011. 21(4): 286-8.
(9) Quintana, L.F., Cofan F., Reverte J.C., et al., Renal cortical necrosis after kidney transplantation associated with the prothrombin 20210A mutation. Nephrol Dial Transplant, 2006. 21(5): 1455-6.
(10) Sidhu, P.S., Cantisani, V., Dietrich, C.F., et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS) in non-hepatic applications. Update 2017. Ultraschall Med, 2018. 39: 2–44.
(11) Kim, H.J., and Cho, O.K., CT scan as an important diagnostic tool in the initial phase of diffuse bilateral renal cortical necrosis. Clin Nephrol, 1996. 45 (2): 125-30.
(12) Fernandez, C.P., Ripolles, T., Martinez M.J., et al., Diagnosis of acute cortical necrosis in renal transplantation by contrast-enhanced ultrasound: a preliminary experience. Ultraschall Med, 2013. 34(4): 340-4.
(13) Harvey, C.J., Alsafi, A., Kuzmich, S., et al., Role of US Contrast Agents in the Assessment of Indeterminate Solid and Cystic Lesions in Native and Transplant Kidneys. Radiographics, 2015. 35(5): 1419-30.
7Figures
Fig 1: Cineclip of the renal transplant graft with colour Doppler demonstrating patent renal artery and vein.
Fig 2: Cineclip of the renal transplant graft with Power Doppler showing apparently good global perfusion.
Fig 3: Cineclip of B-mode images on the left and CEUS on the right showing subcortical rim of hypoperfusion in keeping with cortical necrosis.
Fig 4: Coronal images Balanced Turbo Field Echo (BTFE) MR sequence images demonstrating subtle low signal in the inner cortex.
Fig 5a. and Fig 5b: T1 weight Axial (5a) and Sagittal (5b) images of Contrast enhanced MRA demonstrating lack of peripheral enhancement post Gadolinium administration confirming cortical hypoperfusion and necrosis.
Fig 2: Cineclip of the renal transplant graft with Power Doppler showing apparently good global perfusion.
Fig 3: Cineclip of B-mode images on the left and CEUS on the right showing subcortical rim of hypoperfusion in keeping with cortical necrosis.
Fig 4: Coronal images Balanced Turbo Field Echo (BTFE) MR sequence images demonstrating subtle low signal in the inner cortex.
Fig 5a. and Fig 5b: T1 weight Axial (5a) and Sagittal (5b) images of Contrast enhanced MRA demonstrating lack of peripheral enhancement post Gadolinium administration confirming cortical hypoperfusion and necrosis.


![A rare cause for delayed renal transplant graft function </br> [Jul 2018]](https://efsumb.org/wp-content/uploads/2020/11/july2018-Fig-5b-Cor-Oblique-FS-T1-C-MRI.jpg)