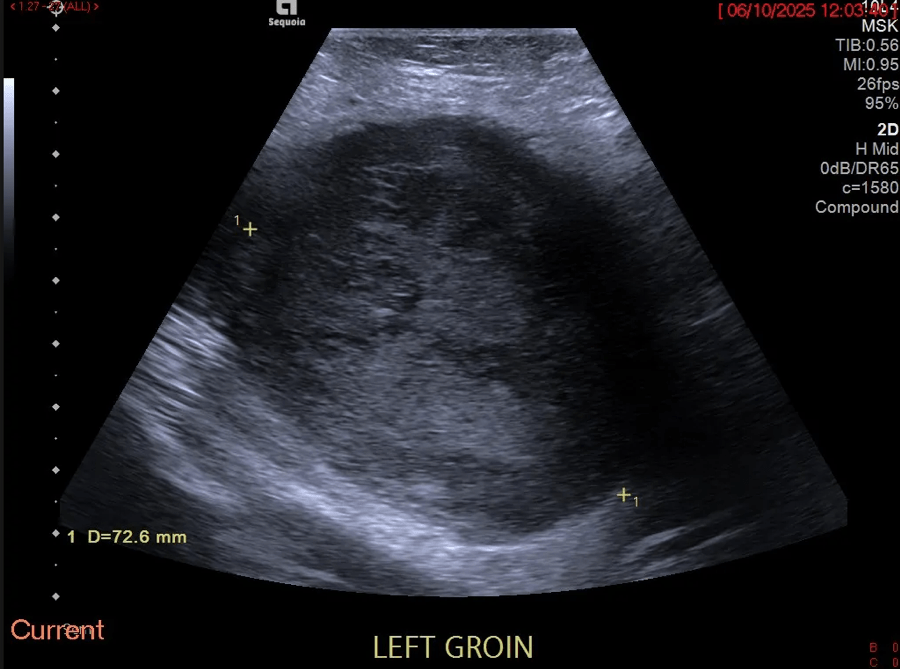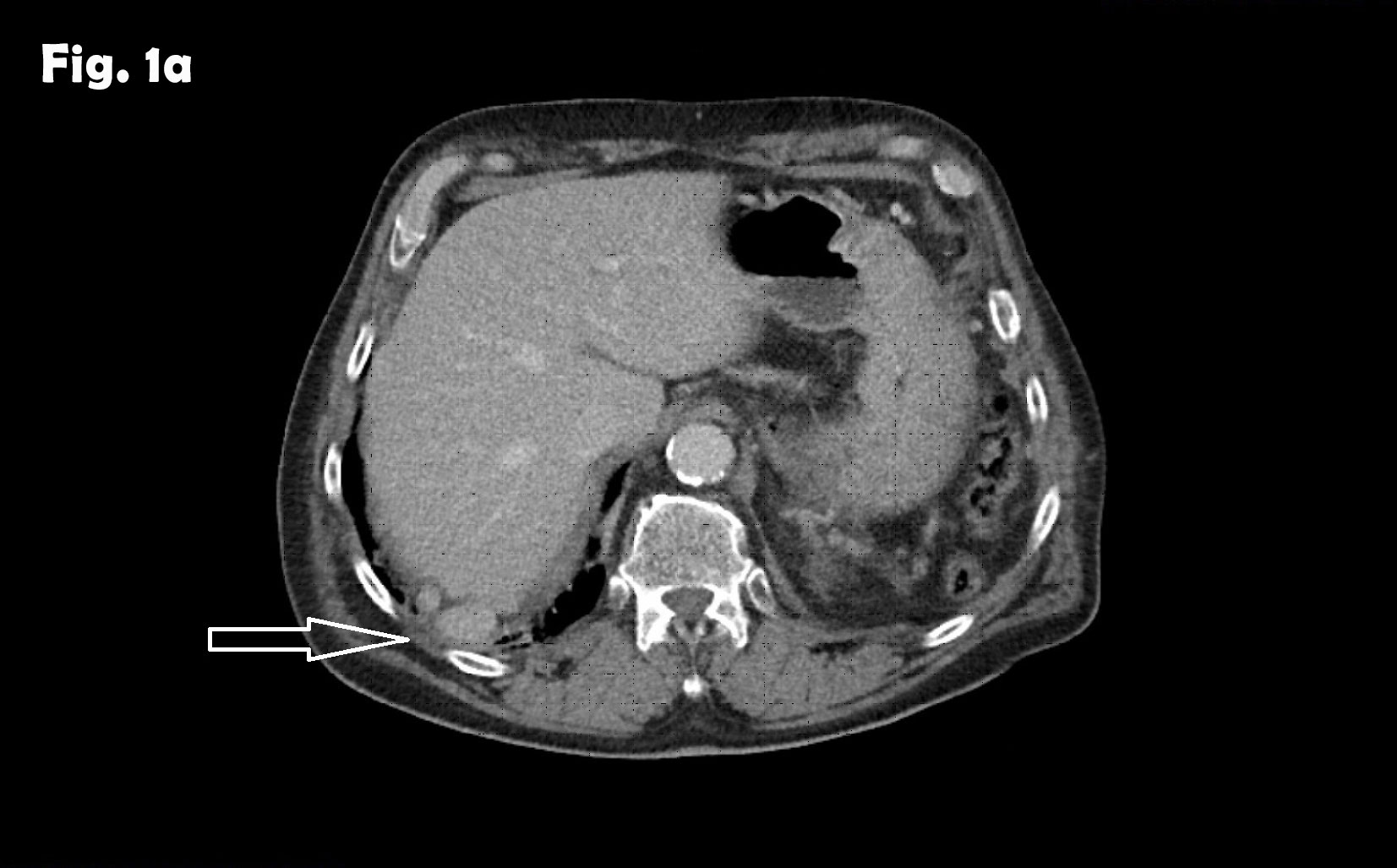
Splenosis mimicking peritoneal metastasis [Sep 2017]
September 11, 2017
Ultrasonographic approach to dysphagia [Nov 2017]
November 12, 2017Multimodal endoscopic ultrasound of a woman with multiple pancreatic lesions
1. Haukeland university hospital, Department of Medicine, Bergen, Norway 2. University of Bergen, Faculty of Odontology and Medicine, Clinical institute 1 (K1) 3. Haukeland university Hospital, Department of Acute- and Gastrointestinal surgery 4. Haukeland University Hospital, Department of Pathology and Gades Institute
A 58-year-old woman previously treated for small-cell pulmonary cancer in 2008 with a curative outcome, was admitted for endoscopic ultrasound with fine-needle aspiration (EUS-FNA) for diagnosing pancreatic lesions. The lesions were discovered on a CT of the abdomen 12 months previously and a follow up MRI had described the lesions as partly cystic. Retrospectively, two lesions were seen in the pancreatic head and tail already in in 2009, with diameters increasing from 3.7-5.6 cm and 1.8-2.5 cm over eight years on cross-sectional imaging. A previous EUS-FNA performed 12 months earlier of the largest lesion in the pancreatic head, provided benign epithelial monolayer cells with no atypical features. In February 2017, a follow-up MRI reported another two small lesions of 8 and 11 mm in addition to the already known lesions, where the lesion located in the pancreatic tail showed a small increase in size. MRI described the lesions as cystic. The patient had during her current stay in hospital also been diagnosed with a late onset type-1 diabetes, with positive titres of Anti IA2 and anti-GAD and she was also being treated for hypothereosis.
The patient was again admitted for EUS and this time we included her in a study with the combination of EUS elastography, contrast enhanced EUS (CE-EUS) with Sonazoid®(GE-Medical) and EUS FNA and/or cyst fluid aspiration. The patient gave her confirmed consent to participate.
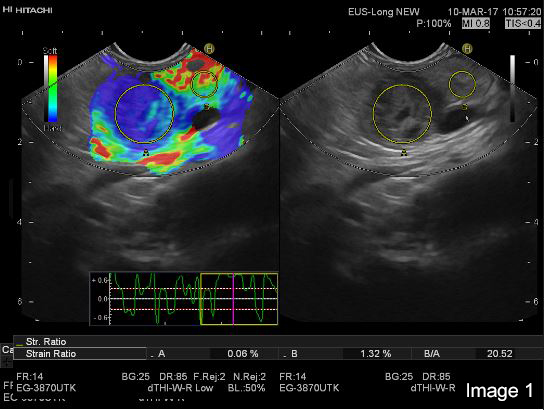
EUS was performed with a Pentax E-3870 UTK linear echoendoscope and Hitachi Ascendus scanner. On B-mode EUS the tumour in the head and tail appeared very similar with a generally hypoechoic inhomogeneous echogenicity pattern and apparently small cystic areas within otherwise solid tumours. Also, the two smaller lesions could be identified in the body and tail of the pancreas. We concentrated the further examination on the lesion in the tail of the pancreas, with a diameter of 25 mm (Image 1 and 2).
On strain based elastography, the tumour tissue was repeatedly imaged as harder than the surrounding pancreatic parenchyma (Image 1 and 2).
On colour-Doppler a “basket sign” was visible with several vessels highlighting the immediate surrounding tissue (Image 3).
After the intravenous administration of 1.7 ml (full vial) of Sonazoid® in a cubital vein, the tumour tissue was visualized as isoechoic compared to surrounding parenchymal pancreatic tissue. The cystic areas within the cystic tumour was shown with clear demarcation to the hypoechoic tumour tissue and were larger than expected by the B-mode image. In the late (venous) phase, particularly visible after 1 min and 20 seconds, the tumour tissue washes out contrast compared to the surrounding pancreatic parenchyma (Image 3).
EUS FNA was performed with a 19 G FNA (Echo-Tip, Cook Medical) needle with up to 10 cm H2O vacuum. Samples were sent on CytoLyt® for cytology and formalin for histology. The cytology showed neuroendocrine cells. The histology gave even more results: Moderately to highly differentiated neuroendocrine tumour, NET-G2, Ki-67: 13%.
Serum Chromogranin A was 1.2 nmol/l, which is in the normal range.
The patient thereafter underwent total pancreatectomy and splenectomy, and the histology from the surgical specimen confirmed the EUS-FNA result: Highly differentiated neuroendocrine tumours (NET-G2) in the pancreas, Ki-67: 8.5% -11.5%, No infiltration in peri-pancreatic fat tissue, 18 lymph nodes without metastases.
Image 2: The hypoechoic tumor tissue in the B mode image is repeatedly corresponding to the blue area indicating harder tissue in the elastogram (left)
Image 3:The hypoechoic tumor tissue in the B mode image is repeatedly corresponding to the blue area indicating harder tissue in the elastogram (left)


![Multimodal endoscopic ultrasound of a woman with multiple pancreatic lesions</br> [Oct 2017]](https://efsumb.org/wp-content/uploads/2020/11/oct2017-image02.jpg)
![Multimodal endoscopic ultrasound of a woman with multiple pancreatic lesions</br> [Oct 2017]](https://efsumb.org/wp-content/uploads/2020/11/oct2017-image03.jpg)