Student Image Challenge 10
July 16, 2019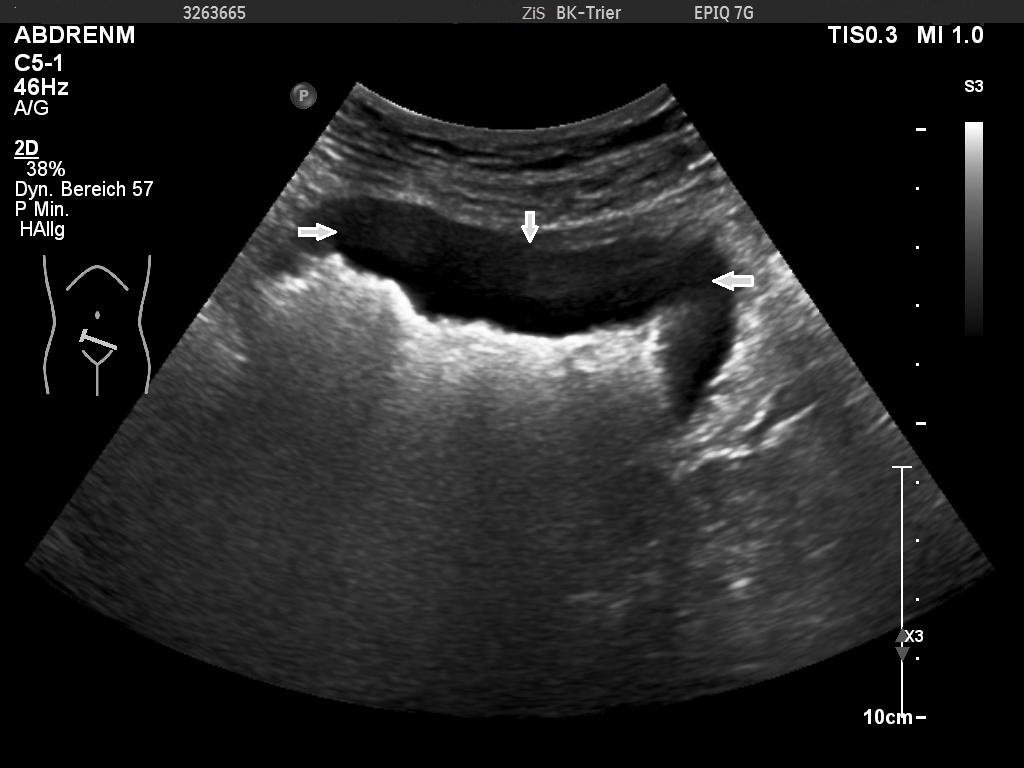
Non Hodgkin lymphoma of the small intestine [Aug 2019]
August 24, 2019The clue is in the bubbles
AUTHORS:
Angus Wilson, Richard Jenkins, Chris Schelvan, Adrian Lim
Angus Wilson, Richard Jenkins, Chris Schelvan, Adrian Lim
1Clinical History
A 45 year-old female was admitted with generalised, non-specific abdominal pain. There white blood cell count was raised (21.5 x109/L) with a neutrophilia (20 x 109/L). An urgent ultrasound scan was requested, to assess for the underlying cause, with a wide differential offered including acute cholecystitis, appendicitis or ovarian pathology.
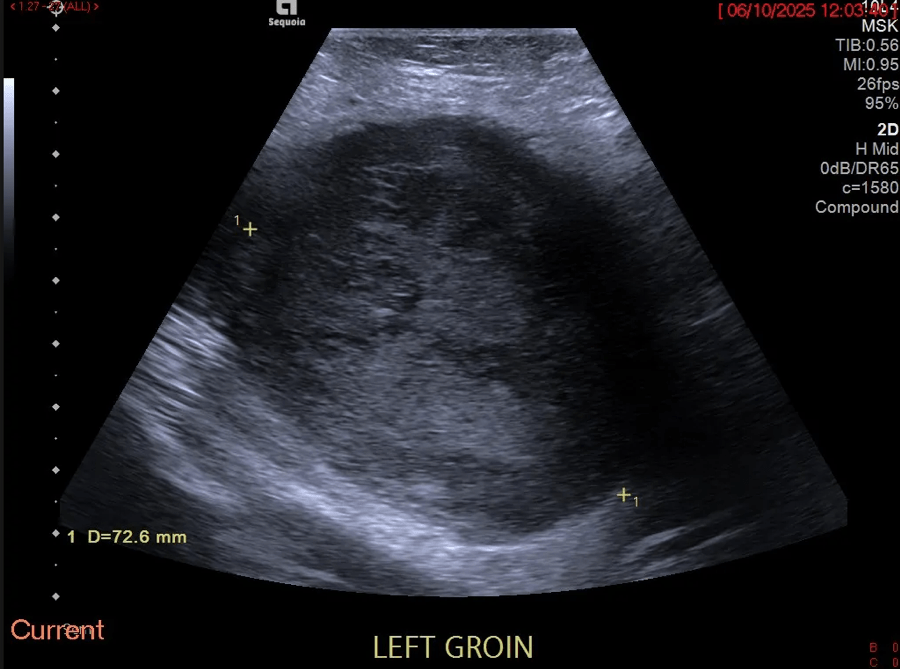
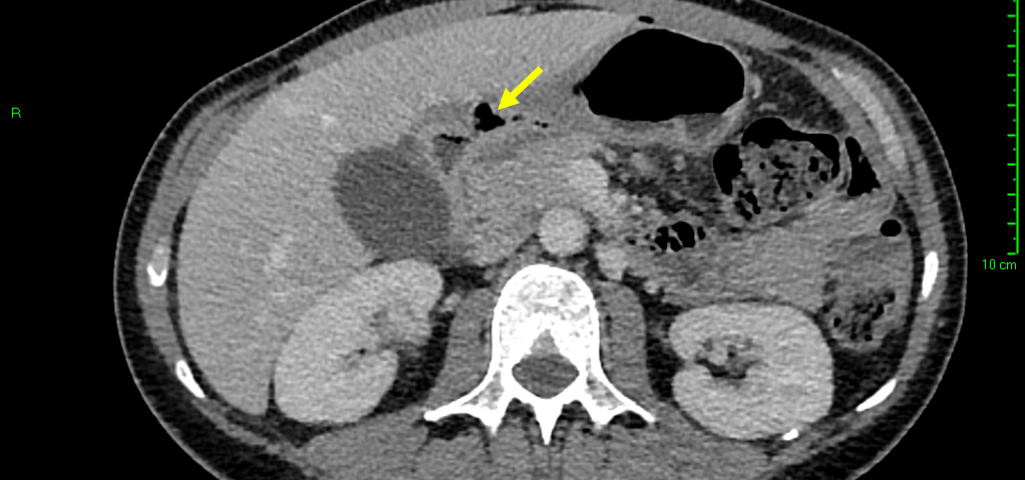
Sonographic examination demonstrated a full thickness mucosal defect of the anterior pre-pyloric region of the stomach, with extraluminal gas locules (see image), trace free fluid in the gallbladder fossa and complex fluid in the right iliac fossa. These features were consistent with a perforated pre-pyloric gastric ulcer. An urgent CT scan was arranged, which confirmed the diagnosis (see images). The patient subsequently underwent emergency laparoscopic surgery. The perforated ulcer, loosely sealed by omentum and the medial edge of the liver, was identified and repaired with an omental plug procedure. Further management included postoperative intravenous antibiotics and PPIs. The patient made a good recovery and was discharged four days post-operatively, with Helicobacter pylori eradication therapy and analgesia.
Sonographic examination demonstrated a full thickness mucosal defect of the anterior pre-pyloric region of the stomach, with extraluminal gas locules (see image), trace free fluid in the gallbladder fossa and complex fluid in the right iliac fossa. These features were consistent with a perforated pre-pyloric gastric ulcer. An urgent CT scan was arranged, which confirmed the diagnosis (see images). The patient subsequently underwent emergency laparoscopic surgery. The perforated ulcer, loosely sealed by omentum and the medial edge of the liver, was identified and repaired with an omental plug procedure. Further management included postoperative intravenous antibiotics and PPIs. The patient made a good recovery and was discharged four days post-operatively, with Helicobacter pylori eradication therapy and analgesia.
2Diagnosis
Perforated anterior pre-pyloric gastric ulcer.
3Discussion
A peptic ulcer is a full-thickness erosion (more than 5mm) of the gastric or duodenal mucosa, extending into the submucosa. The anterior duodenal bulb and gastric pre-pylorus are the most common locations of ulceration, and reflect the most common sites of peptic ulcer perforation [1].
Perforated peptic ulcer is variable in clinical presentation, and may mimic other causes of the acute abdomen, such as acute cholecystitis, pancreatitis, appendicitis or ruptured abdominal aortic aneurysm [2, 3]. It has been suggested that perforated peptic ulcer is not considered in the preliminary differential diagnosis in up to 20% of proven cases [1]. Given that the condition is associated with a short term mortality rate of up to 20-30%, early recognition in order to expedite surgical intervention is of critical importance [3, 4].
Ultrasound assessment is not the imaging modality of choice for a perforated peptic ulcer, however in cases of vague abdominal pain and an ultrasound is the first imaging test, this case serves as a reminder that such diagnoses should be considered and can occasionally be picked by ultrasound. There have been similar reports by a number of authors [5, 6, 7] and it is therefore of paramount importance that sonographers and radiologists are aware of the characteristic sonographic appearances of perforated peptic ulcer; the features of which may be direct or indirect [8].
Direct
An active peptic ulcer may be visualised as a localised, asymmetric thickening of the gastric or duodenal wall, with or without echogenic gas in the ulcer crater. A perforated peptic ulcer may be seen directly as a whole-thickness defect, with an echogenic linear track of gas locules originating from the ulcer crater and extending extraluminally into the peritoneal cavity [2, 8].
Indirect
Indirect signs include localised extraluminal gas, pneumoperitoneum, fluid collections and inflammatory fatty change. Extraluminal gas locules accumulate close to the site of perforation, and may be evident even in small volumes; visualisation should promote further focused examination of the region. Pneumoperitoneum may be significant, with free intraperitoneal air collecting in the perigastric, periduodenal and/or perihepatic spaces. Free fluid may be present in variable volume; in some cases limited locally to the perforation site, and in others present in larger volume, extending to the perihepatic space, along the right paracolic gutter and into the pelvis. Fatty inflammatory change local to an active ulcer may also provide subtle indirect evidence of peptic perforation [8].
Perforated peptic ulcer is variable in clinical presentation, and may mimic other causes of the acute abdomen, such as acute cholecystitis, pancreatitis, appendicitis or ruptured abdominal aortic aneurysm [2, 3]. It has been suggested that perforated peptic ulcer is not considered in the preliminary differential diagnosis in up to 20% of proven cases [1]. Given that the condition is associated with a short term mortality rate of up to 20-30%, early recognition in order to expedite surgical intervention is of critical importance [3, 4].
Ultrasound assessment is not the imaging modality of choice for a perforated peptic ulcer, however in cases of vague abdominal pain and an ultrasound is the first imaging test, this case serves as a reminder that such diagnoses should be considered and can occasionally be picked by ultrasound. There have been similar reports by a number of authors [5, 6, 7] and it is therefore of paramount importance that sonographers and radiologists are aware of the characteristic sonographic appearances of perforated peptic ulcer; the features of which may be direct or indirect [8].
Direct
An active peptic ulcer may be visualised as a localised, asymmetric thickening of the gastric or duodenal wall, with or without echogenic gas in the ulcer crater. A perforated peptic ulcer may be seen directly as a whole-thickness defect, with an echogenic linear track of gas locules originating from the ulcer crater and extending extraluminally into the peritoneal cavity [2, 8].
Indirect
Indirect signs include localised extraluminal gas, pneumoperitoneum, fluid collections and inflammatory fatty change. Extraluminal gas locules accumulate close to the site of perforation, and may be evident even in small volumes; visualisation should promote further focused examination of the region. Pneumoperitoneum may be significant, with free intraperitoneal air collecting in the perigastric, periduodenal and/or perihepatic spaces. Free fluid may be present in variable volume; in some cases limited locally to the perforation site, and in others present in larger volume, extending to the perihepatic space, along the right paracolic gutter and into the pelvis. Fatty inflammatory change local to an active ulcer may also provide subtle indirect evidence of peptic perforation [8].
4Clinical Importance
This case illustrates the importance of perforated peptic ulcer as a cause of acute non-specific abdominal pain, with characteristic sonographic appearances. These features may be direct or indirect, and subtle; careful consideration must be given to scanning technique in order to facilitate recognition. Understanding of the sonographic appearances of perforated peptic ulcer may aid earlier diagnosis, and thus expedite further imaging and/or surgical intervention.
5Teaching Points
• Perforated peptic ulcer is a surgical emergency
• The radiologist/sonographer should consider perforated peptic ulcer in the differential diagnosis in the patient presenting for ultrasound assessment with acute non-specific abdominal pain
• In the patient undergoing ultrasound assessment for non-specific abdominal pain, a complete ultrasound examination of the abdomen, including assessment of the stomach and duodenum, is recommended.
• Look for direct signs of peptic ulcer perforation, including a full-thickness gastric or duodenal wall defect with an associated echogenic linear array of gas locules
• Look for indirect signs of peptic ulcer perforation, including localised extraluminal gas, pneumoperitoneum, and abdominopelvic fluid collections.
• The radiologist/sonographer should consider perforated peptic ulcer in the differential diagnosis in the patient presenting for ultrasound assessment with acute non-specific abdominal pain
• In the patient undergoing ultrasound assessment for non-specific abdominal pain, a complete ultrasound examination of the abdomen, including assessment of the stomach and duodenum, is recommended.
• Look for direct signs of peptic ulcer perforation, including a full-thickness gastric or duodenal wall defect with an associated echogenic linear array of gas locules
• Look for indirect signs of peptic ulcer perforation, including localised extraluminal gas, pneumoperitoneum, and abdominopelvic fluid collections.
6References
1. Thorsen K., Glomsaker T., von Meer A., Søreide K. & Søreide JA. (2011) Trends in diagnosis and surgical management of patients with perforated peptic ulcer. Journal of Gastrointestinal Surgery. 15(8):1329–1335.
2. Puylaert J.(2003). Ultrasonography of the acute abdomen: gastrointestinal conditions. Radiologic Clinics of North America. 41. 1227-42, vii.
3. Søreide K., Thorsen K., Harrison E., Bingener J., Møller M., Ohene-Yeboah M. & Søreide J. (2015) Perforated peptic ulcer. Lancet. 386(10000):1288–1298.
4. Møller M., Adamsen S., Thomsen R. & Møller A. (2011) Multicentre trial of a perioperative protocol to reduce mortality in patients with peptic ulcer perforation. British Journal of Surgery. 98(6):802–10.
5. Tsai K., Wang H., Huang G. & Wang S. (1998) Preoperative sonographic diagnosis of sealed-off perforated gastric ulcer. Journal of Clinical Ultrasound; 26:269–271.
6. Ioannis M., Dimitra Z., Hristos H. & Ioannis D. (2006) Peptic ulcer perforation: sonographic imaging of active fluid leakage. Journal of Clinical Ultrasound. 34(7):365.
7. Fujii Y., Asato M., Taniguchi N., Shigeta k., Omoto K., Itoh K. & Suzukawa M. (2003) Sonographic diagnosis and successful nonoperative management of sealed perforated duodenal ulcer. Journal of Clinical Ultrasound; 31:55–58.
8. Kuzmich S., Harvey C., Fascia D., Kuzmich T., Neriman D., Basit R. & Tan K. (2012) Perforated Pyloroduodenal Peptic Ulcer and Sonography. American Journal of Roentology. 199(5):W587-94.
2. Puylaert J.(2003). Ultrasonography of the acute abdomen: gastrointestinal conditions. Radiologic Clinics of North America. 41. 1227-42, vii.
3. Søreide K., Thorsen K., Harrison E., Bingener J., Møller M., Ohene-Yeboah M. & Søreide J. (2015) Perforated peptic ulcer. Lancet. 386(10000):1288–1298.
4. Møller M., Adamsen S., Thomsen R. & Møller A. (2011) Multicentre trial of a perioperative protocol to reduce mortality in patients with peptic ulcer perforation. British Journal of Surgery. 98(6):802–10.
5. Tsai K., Wang H., Huang G. & Wang S. (1998) Preoperative sonographic diagnosis of sealed-off perforated gastric ulcer. Journal of Clinical Ultrasound; 26:269–271.
6. Ioannis M., Dimitra Z., Hristos H. & Ioannis D. (2006) Peptic ulcer perforation: sonographic imaging of active fluid leakage. Journal of Clinical Ultrasound. 34(7):365.
7. Fujii Y., Asato M., Taniguchi N., Shigeta k., Omoto K., Itoh K. & Suzukawa M. (2003) Sonographic diagnosis and successful nonoperative management of sealed perforated duodenal ulcer. Journal of Clinical Ultrasound; 31:55–58.
8. Kuzmich S., Harvey C., Fascia D., Kuzmich T., Neriman D., Basit R. & Tan K. (2012) Perforated Pyloroduodenal Peptic Ulcer and Sonography. American Journal of Roentology. 199(5):W587-94.


![The clue is in the bubbles </br> [Jul 2019]](https://efsumb.org/wp-content/uploads/2020/11/jul2019-fig1.png)
![The clue is in the bubbles </br> [Jul 2019]](https://efsumb.org/wp-content/uploads/2020/11/jul2019-fig2.png)
![The clue is in the bubbles </br> [Jul 2019]](https://efsumb.org/wp-content/uploads/2020/11/jul2019-fig3.png)