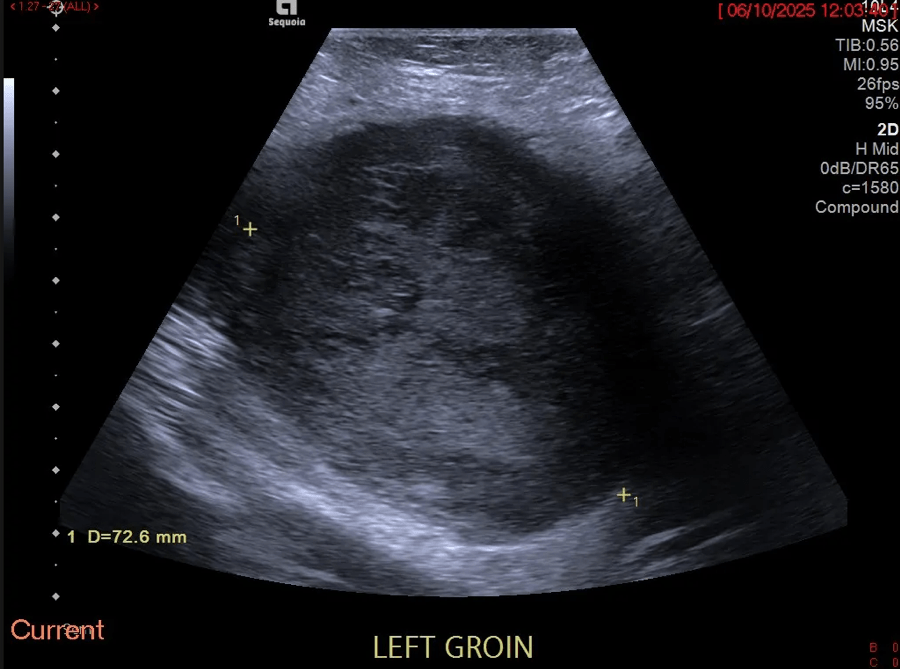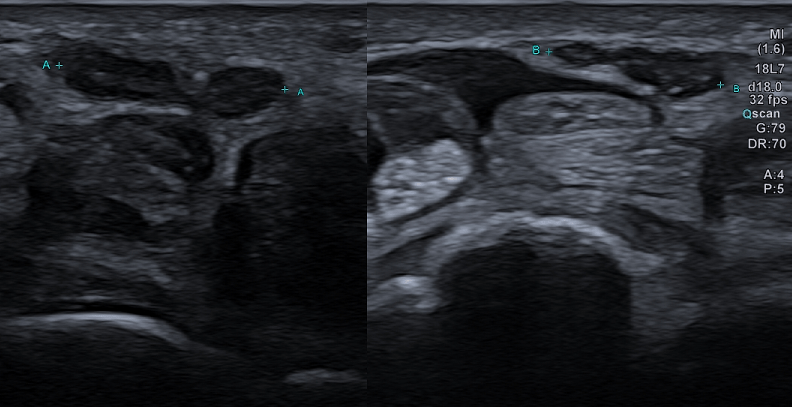
Bifid median nerve with persistent median artery in carpal tunnel syndrome: assessment with US [Nov 2022]
January 12, 2023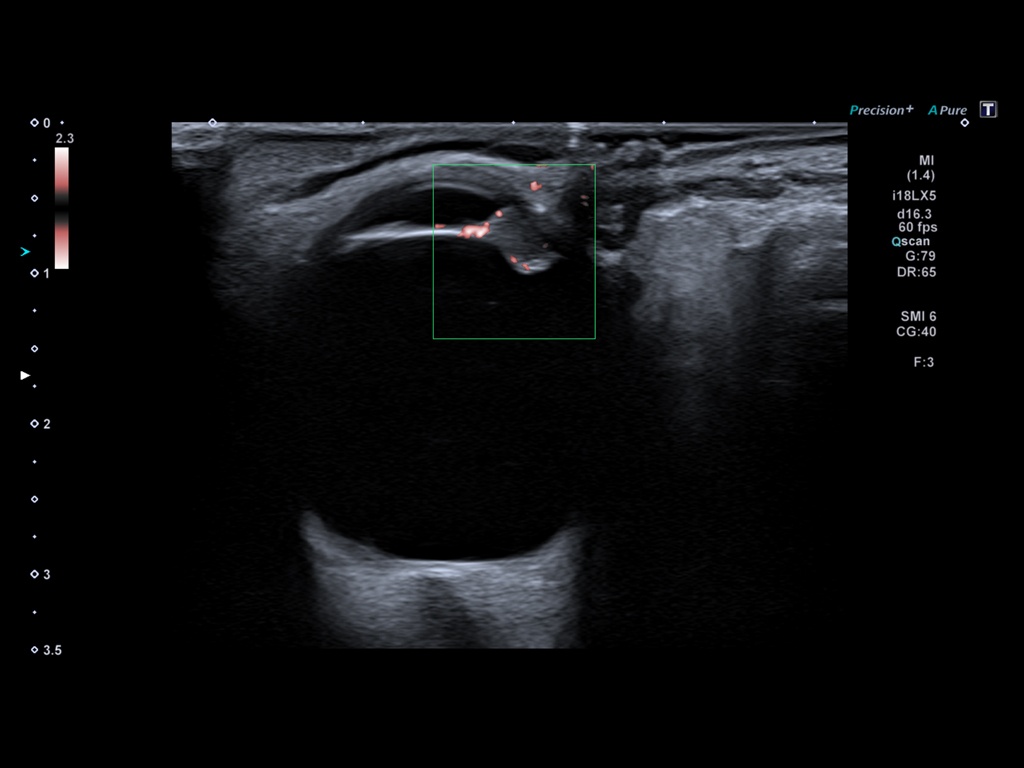
An Eye for an Eye [Jan 2023]
April 17, 2023SUBMIT YOUR CASE
EFSUMB invites submission of interesting cases for the website section 'Case of the Month'. All CoM submissions are eligible for selection for free registration at the next Euroson congress. Two cases that receive the most 'likes' in a year will receive free registration for the next EUROSON congress and the third most liked liked case will receive a cash prize of 100 EUR.
2022 Winners were:
Top rated is Sonographic Diagnosis of a Pott’s Puffy Tumor in a 5-year-old-girl - [Jul 2022]
M Brandt Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital of Regensburg, Germany
Second is Ischemic Colitis as Complication of SARS-CoV-2 Infection - [June 2022]
Federica Lepore Department of Internal Medicine, San Matteo Hospital Foundation, University of Pavia, Italy
Both winners received free registration at EUROSON2023.
View Submission Template
Ultrasound diagnosis of bladder endometriosis
Sergiu Puiu
State University of Medicine and Pharmacy “Nicolae Testemitanu”, Chisinau, Republic of MoldovaE- mail: puiusv@yahoo.com
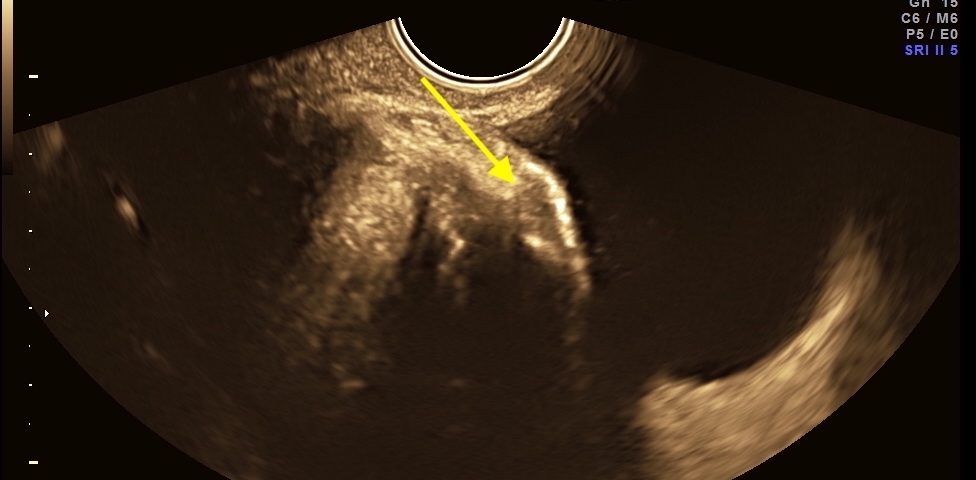
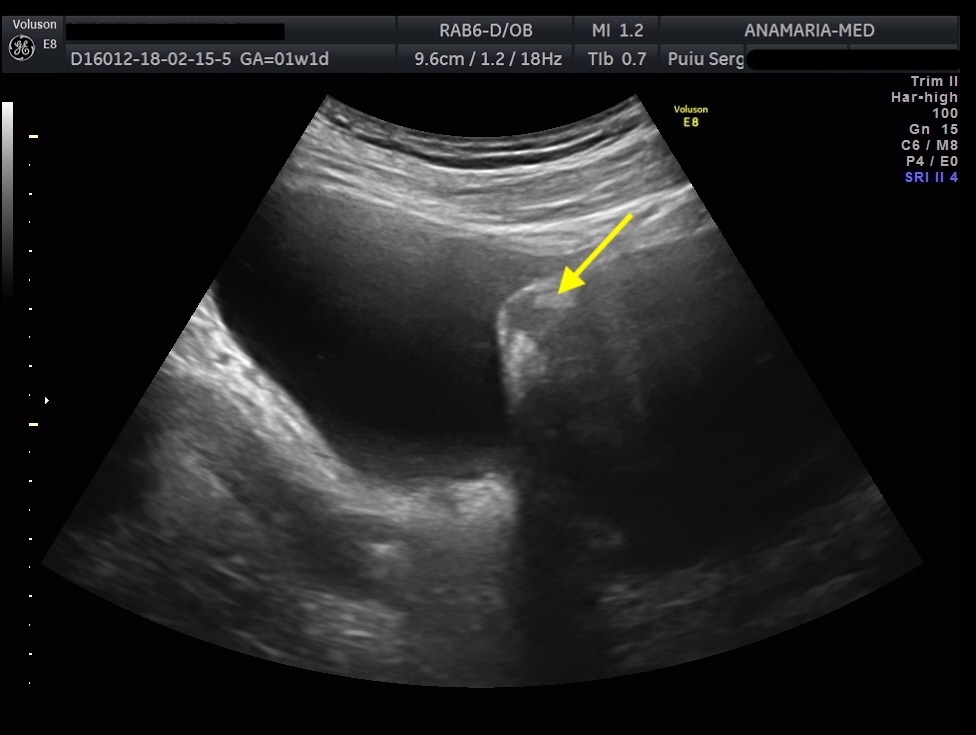
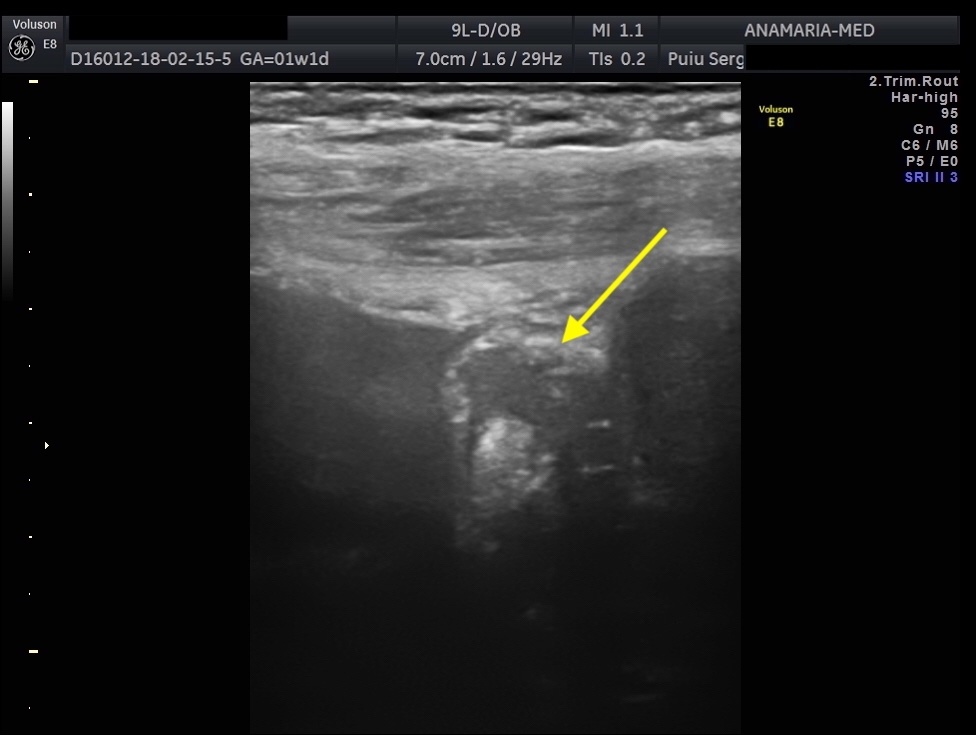
Endometriosis is a chronic condition that affects up to 10% of people assigned female at birth, leading to pain, infertility and reduced quality of life. The two phenotypes are superficial endometriosis (SE) and deep endometriosis (DE). Endometriosis of the urinary system accounts for less than 1% of all endometriosis. However, the real prevalence remains unclear, since around 50% of affected women may be asymptomatic. Diagnosing both types remains challenging, resulting in diagnostic delays for patients. Bladder endometriosis is defined as endometriosis infiltrating the detrusor muscle, more frequently the bladder base and bladder dome, less the trigonal zone and extra-abdominal bladder and it is the most common, representing 85% of urinary tract endometriosis. Typically, women with bladder endometriosis present with cyclical and non-cyclical pelvic pain, dysuria, but may also have urinary frequency, recurrent urinary tract infections and hematuria, and, more atypically, urinary incontinence.
CLINICAL PERSPECTIVE:
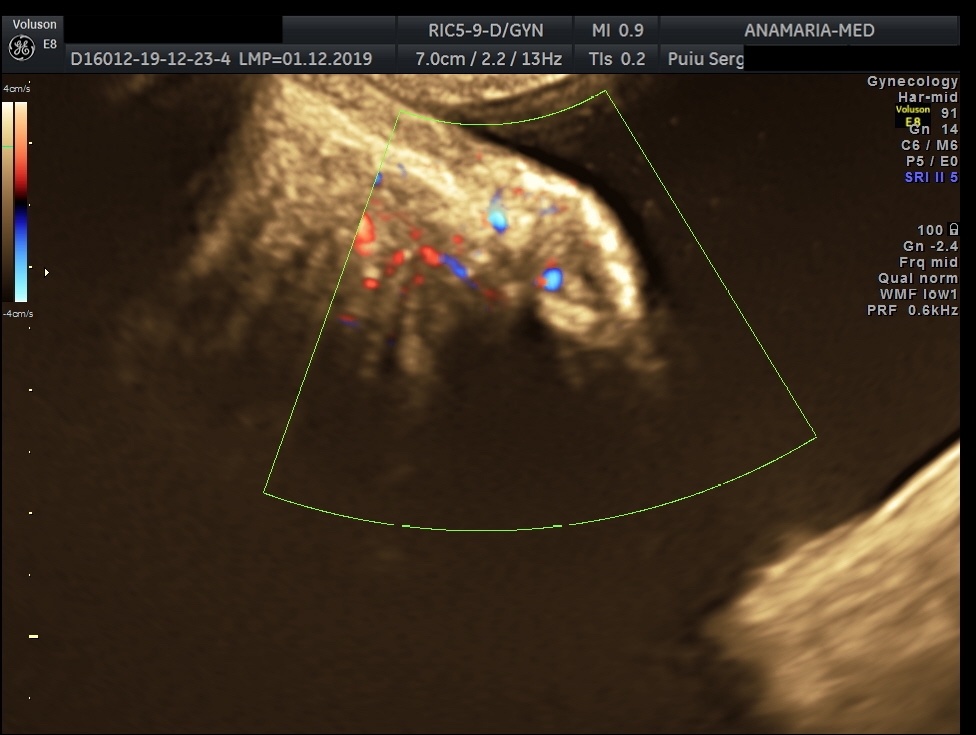
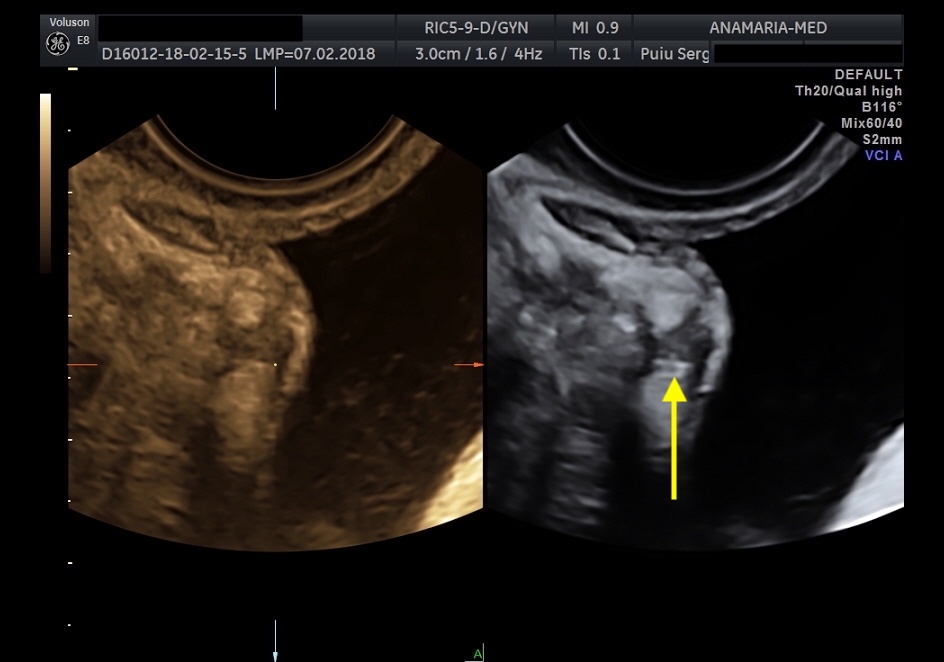
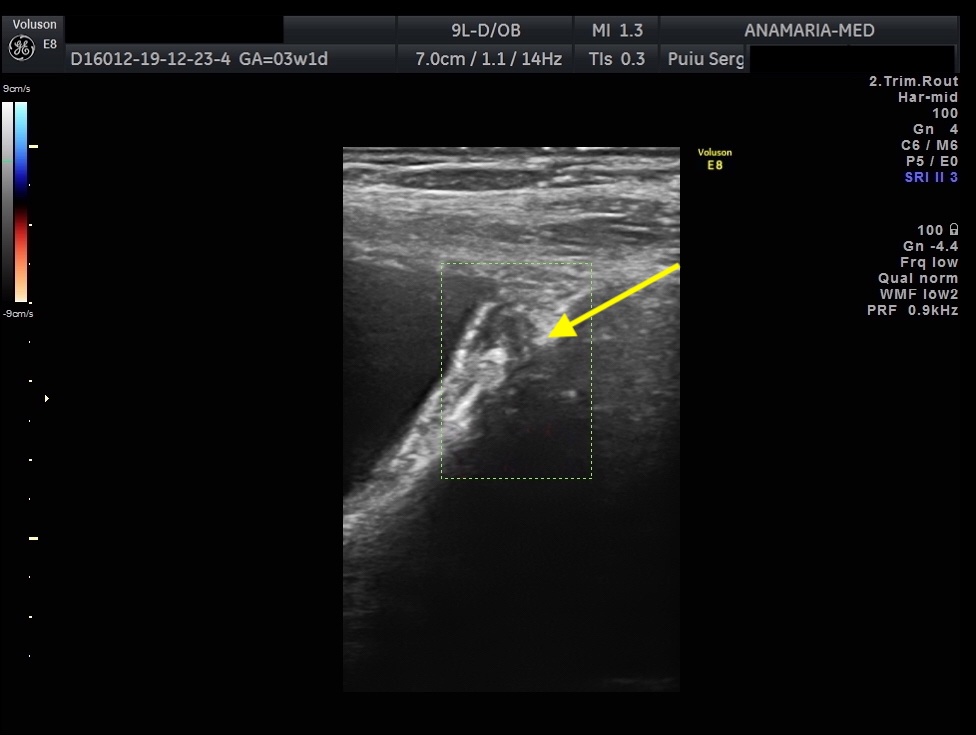
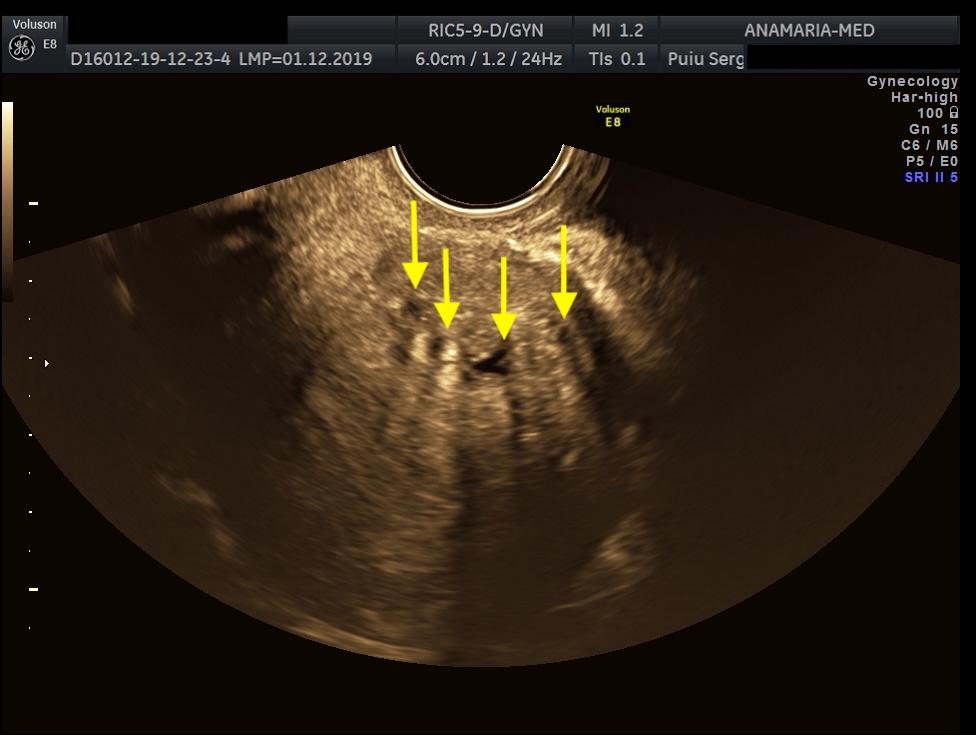
The initial evaluation of suspected bladder endometriosis includes the medical history, a physical examination, and complementary tests (laboratory testing, cystourethroscopy and imaging techniques). Ultrasound is an accurate tool to diagnose urinary tract involvement in women with suspected pelvic endometriosis. A moderately filled bladder allows better assessment of bladder wall and endometriotic nodules detection. Common ultrasound features are hypoechoic linear or nodular nodules, with or without regular contours involving the muscularis (most common) or submucosa of the bladder. Usually, endometriotic lesions are poorly or no vascularized. Bladder endometriosis is diagnosed only if the muscularis of the bladder wall is affected; lesions involving only the serosa represent superficial disease. Pelvic organ mobility and obliteration of the uterovesical region can be evaluated using the “sliding sign”. However, adhesions in the anterior pelvic compartment are present in nearly one third of women with a previous Cesarean section and are not necessarily a sign of pelvic endometriosis. The cystourethroscopy may be helpful to confirm the diagnosis. Cystoscopically, bladder endometriosis can have a spectrum of possible appearances from normal-appearing mucosa that is noticeably raised due to a nodule beneath the mucosa to infiltration through the mucosa. In the latter scenario, lesions can appear to be multiloculated with a combination of colors (from the same color as the bladder mucosa to a blue/violet color).
THERAPY PLANNING:
Treatment can be expectant, medical or surgical. Hormonal therapy is an effective option for those who are not planning to conceive or to undergo surgery. For patients with pain symptoms due to bladder endometriosis, continuous progesterone-based regimens (pills, intrauterine device, implant, injection), combined estrogen-progesterone therapy (continuous or sequential regimens) and gonadotropin-releasing hormone agonist (with or without add-back therapy) have all been associated with an improvement of symptoms of bladder endometriosis. Specifically, Dienogest may be suitable for patients refusing surgery, considering the effectiveness and tolerance for long-term use. Segmental bladder resection/partial cystectomy is the bladder-preserving surgery and offers the complete removal of bladder endometriotic nodules.
OUTCOME & PROGNOSIS:
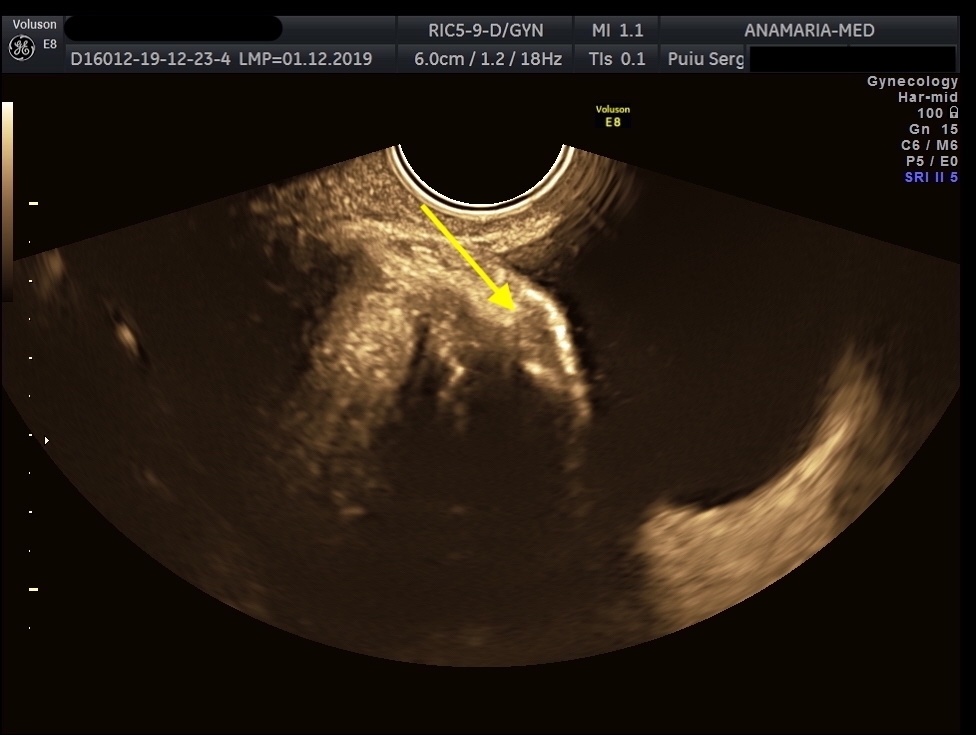
Women who respond to medical management can continue the treatment until menopause or until the desire to conceive from pregnancy or to achieve an optimal quality of life and reduce the risk of progression. The mentioned patient accepted conservative management with a sonographic follow-up. Dienogest administration was effective and tolerable in alleviating urinary symptoms. The size of the endometriotic nodule lesion remain stable during Dienogest administration, and the effect was maintained until 36 months thereafter.
Ultrasound is more than just a diagnostic test as it can help patients and clinicians to understand the extent and location of endometriosis by interpreting real-time tenderness and mobility, as well as facilitate objective monitoring of disease over time and in many cases is helpful in planning a multidisciplinary surgical approach
2. Maggiore U.L.R., Ferrero S., Candiani M., et al: A systematic review of pathogenesis, diagnosis, treatment, impact on fertility, and risk of malignant transformation. Eur. Urol. 2017; 71: 790–807.
3. Savelli L, Manuzzi L, Pollastri P, Mabrouk M, Seracchioli R, Venturoli S. Diagnostic accuracy and potential limitations of transvaginal sonography for bladder endometriosis. Ultrasound Obstet Gynecol 2009; 34: 595–600.
4. Geng J.-H., Lee Y.-C. Bladder Endometriosis. N. Engl. J. Med. 2019;381:e43.
5. Leonardi M, Espada M, Kho RM, et al. Endometriosis and the Urinary Tract: From diagnosis to surgical treatment. Diagnostics (Basel). 2020; 10: 771.
6. Knabben L, Imboden S, Fellmann B, Nirgianakis K, Kuhn A, Mueller M.D. Urinary tract endometriosis in patients with deep infiltrating endometriosis: Prevalence, symptoms, management, and proposal for a new clinical classification. Fertil. Steril. 2015;103:147–152.